Research Article
Profile of vitamin D receptor polymorphism Bsm I and FokI in end stage renal disease Egyptian patients on maintenance hemodialysis
EL-Attar HA1*, Mokhtar MM2 and Gaber EW3
1Professor of Chemical Pathology, Medical Research Institute, University of Alexandria, Egypt
2Professor of Human Genetics, Medical Research Institute, Alexandria University, Egypt
3Professor of Internal Medicine, Medical Research Institute, Alexandria University, Egypt
*Address for Correspondence: EL-Attar HA, Professor of Chemical Pathology, Medical Research Institute, University of Alexandria, Egypt; Tel: 01020244286; Email: [email protected]
Dates: Submitted: 10 August 2017; Approved: 28 August 2017; Published: 30 August 2017
How to cite this article: EL-Attar HA, Mokhtar MM, Gaber EW. Profile of vitamin D receptor polymorphism Bsm I and FokI in end stage renal disease Egyptian patients on maintenance hemodialysis. J Clini Nephrol. 2017; 1: 026-040. DOI: 10.29328/journal.jcn.1001005
Copyright License: © 2017 EL-Attar HA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic renal failure; End stage renal disease (ESRD); Hemodialysis; Gene polymorphism; Vitamin D receptor polymorphism; Vdr; Bsmi; Foki
Abstract
Objective: In end stage renal disease, the synthesis of vitamin D is disturbed.Hyperparathyroidism is one of the key factors in the pathogenesis of many of the complications of dialysis mainly bone and cardiovascular complications.Aim:This study aimed at assessing vitamin D receptor gene polymorphisms BsmIand FokI in Egyptian patients with end stage renal disease on maintenance haemodialysis and the assosciation of these polymorphisms with cardiovascular complications and hyperparathyroidism among these patients.
Methods: One hundred subjects, recruited from Medical Research Institute, from March to July 2014, divided into two main groups; the control group which included thirty apparently healthy subjects and the patients group which included seventy patients with end stage renal disease on maintenance haemodialysis with median 4 years. To all studied subjects, detailed history was taken, thorough physical examination, carotid intima media thickness, presence of plaques and ECG ischemic changes. Laboratory investigations included serum levels of: glucouse, urea, creatinine, uric acid, albumin, total cholesterol, low and high density lipoproteins, calcium, phosphorus, and CRP as well as plasma PTH level. For molecular studies, the detection of BsmI and FokI polymorphisms using polymerase chain reaction and restriction fragment length polymorphism (PCR / RFLP) technique.
Results: 1.No statistically significant difference could be detected in both BsmI and FokI gene polymorphisms between the hemodialysis patients and the controls, suggesting that the development of ESRD had no relation with either VDR BsmI or FokI gene polymorphisms.2.No statistically significant difference were found in these polymorphisms between the hemodialysis patients with or without cardiovascular complications or between patients with PTH level less or more than 300 pg/ml. These results suggest that the development of cardiovascular complications and secondary hyperparathyroidism among Egyptian patients on maintenance haemodialysis cannot be attributed to these two gene polymorphisms.
Conclusion: No association could be found between the variant alleles of BsmI and FokI gene polymorphisms and the development of ESRD, cardiovascular complications and secondary hyperparathyroidism among the studied samples of Egyptian patients on maintenance haemodialysis.
Introduction
Vitamin D is a group of fat soluble steroids that primarily maintain normal blood levels of calcium and phosphorus [1].The active metabolite (1,25-dihydroxyvitamin D3) is essential in bone and mineral homeostasis and it is a mediator of cell proliferation, differentiation and immune regulation [2,3]. Vitamin D promotes parathyroid hormone (PTH) and works with PTH in directing the mobilization of calcium and phosphorus from bone to normalize blood calcium levels. The actions of vitamin D are mediated by both genomic and non-genomic pathways. The genomic pathways are activated by the binding of 1, 25(OH)2D3 to nuclear vitamin D receptor (VDR). The non-genomic pathways are activated via cell membrane receptors for vitamin D, and might be responsible for the rapid effects of the hormone [4]. However, most of the biological actions of vitamin D are believed to be exerted through the nuclear VDR mediated control of target genes [5].
Vitamin D receptor (VDR) is a member of the nuclear receptor superfamily of transcription factors, and regulates gene expression in a ligand-dependent manner [6]. VDRs are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content [7].
The VDR is a member of the superfamily of nuclear hormone receptors (NHRs) [8]. In humans, the VDR protein consists of 427 amino acids, with a molecular mass of ~48 kDa.
The human vitamin D receptor gene (hVDR) is located on the long (q) arm of chromosome 12 at position 13.11 [9].
The VDR can be divided by function into several domains. VDR domains are defined as the N-terminal A/B domain, region C or the DNA-binding domain (DBD), the hinge region, and the multifunctional ligand-binding domain [10].
A polymorphism is a genetic variant that appears in at least 1% of the population. These changes can occur in non-coding parts of the gene (introns), so they would not be seen in the protein product. Changes in the regulatory parts of the gene would then affect the degree of expression of the gene, and thus the levels of the protein. For instance, changes in the 5′-promoter of the VDR gene can affect mRNA expression patterns and levels, while 3′ untranslated region (UTR) sequence variations can affect the mRNA stability and protein translation efficiency. However, the changes can take place in exonic parts of the DNA, then leading to changes in the protein sequence. Nonetheless, changes in exonic sequences of the DNA which do not alter the protein structure are also possible, and are called synonymous polymorphisms [11].
These changes usually create or abolish sites for restriction enzymes to cut the DNA. Digestion with the enzyme then produces DNA fragments of a different length which can be detected by electrophoresis. These polymorphisms are called Restriction Fragment Length Polymorphisms (RFLPs). A single nucleotide polymorphism (SNP) is a site on the DNA in which a single base pair varies from person to person [12]. SNPs have gained popularity in recent years and are touted as the genetic markers of choice for the study of complex genetic traits [13]. Because of their abundance in the human genome as well as their high frequencies in the human population, polymorphisms have often been studied with the aim of explaining variations in the risk for common diseases [14].
Several common genetic variants have been identified in the VDR gene. Most of these variants, including the FokI(‘f’), BsmI (‘b’), Tru9I (‘u’), EcoRV, ApaI(‘a’), and TaqI(‘t’) polymorphisms, are identified by a biallelic variation in a restriction endonuclease site and are named based on the restriction endonuclease [15].
The BsmI polymorphism is unlikely to have functional consequences as this variants is located in intron 8. BsmI does not alter the amount, structure, or function of the final VDR protein produced, but it is strongly linked with a poly (A) repeat and may affect VDR messenger RNA stability [16]. It has also been reported that the b alleles appear to be more active than the B alleles. According to the SNP database VDR BB, Bb, and bb genotypes are referred to as AA, AG, and GG. The size of the PCR product for the BsmI polymorphism was 825 bp. Following the digestion, two restriction fragments of 650 and 175 bp were observed for GG homozygotes, a single 825 bp band was obtained for AA homozygotes, and AG individuals displaying all three bands [9], the genotype frequency of BsmI (rs: 1544410) among Caucasians was found to be: (BB):(Bb): (bb)=22.1 : 43.4 : 34.5 [17].
Exon 2 contains the FokI polymorphism, a T/C transition polymorphism (ATG to ACG) occurs at the first of two potential translation initiation sites and results in an alteration of the VDR protein structure [18].
Individuals with the T allele (designated f, indicating the presence of the FokI, initiate translation at the first ATG site to synthesize the full-length (427 amino acid) VDR protein. Individuals with the C allele (designated F, indicating the absence of the FokI site) .These individuals lack the three NH2- terminal amino acids of the full-length VDR protein, resulting in a protein of only 427 amino acids [16,18]. Thus, Ff and ff genotypes express the full-length VDR isoform, while the FF genotype expresses the shorter isoform. The F alleles of the FokI polymorphism appear to be more transcriptionally active than the f alleles .According to population based allele frequencies of disease associated polymorphisms in the Personalized Medicine Research Project which was published in 2010 [19], the minimum allele frequency (MAF) of FokI (rs: 2228570) among Caucasians was found to be: (FF) : (Ff) : (ff)=37.2 : 43.4 : 19.5
The discovery of genetic variants linked with susceptibility of diseases can be the key to advances in preventive medicine.If a relationship with the disease emerges from association studies, this finding would strongly support the idea that the candidate gene is in some way involved in the disease [11].
Chronic kidney disease (CKD) is a modern day global epidemic and is now recognized as a public health issue.Disturbance in mineral and bone metabolism accompanied by soft tissue and vascular calcification is one of the most common and important consequences of CKD development and progression. Mineral and bone disorders are common in CKD and are collectively referred to as CKD- mineral and bone disorder [20].
Chronic kidney failure is also referred to as end-stage renal disease (ESRD).Some studies reported that about 40% of patients with ESRD undergoing heamodialysis developed cardiovascular diseases (CVD). In 8.8% of them, death occurred, of these deaths 63.6% were due to CVD [20,21].
Secondary hyperparathyroidism (sHPT) describes a complex alteration in bone and mineral metabolism that occurs as a direct result of chronic kidney disease (CKD) [20]. Identifying patients at risk and evaluating for sHPT is imperative because early intervention may slow or arrest the progression of both bone and cardiac diseases. Dietary concerns, pharmacotherapy, and patient adherence are all important considerations in creating a successful treatment plan [22].
The FokI and BsmIpolymorphisms of the vitaminD receptor gene are regarded as strong markers of disturbed of vitamin D signaling pathway [23].
Some reports showed a relationship between BsmI polymorphism and primary HPT. These results led to hypothesize that VDR polymorphisms could be involved in sHPT due to chronic renal failure [11].
Some studies have been conducted in chronic renal failure and hemodialysis patients in order to investigate any relation between PTH levels and VDR polymorphisms and it was found that the allele B of the VDR BsmI polymorphism has been associated with protection against end stage renal failure. Also, BsmI gene polymorphism seemed to be associated with PTH function; as levels of the intact PTH were found lower in patients with BB genotype than those with Bb or bb genotypes [23,24].
It has been suggested that FokI polymorphism of VDR gene may determine PTH response in chronic renal failure patients as serum PTH level was often found to be higher in CRF patients having FF genotype than in those having Ff or ff genotypes [25].
The effect of VDR polymorphisms is one of the growing field of research due to the complex role played by vitamin D in chronic renal disease patients [11].
Regarding insufficient studies in the literature about CRDand VDRpolymorphisms, a lot is still needed to explore the complex relation between CRF, PTH, vitamin D and VDR polymorphisms.
Aim
The present study aimed at assessing vitamin D receptor gene polymorphisms BsmI and FokI in end stage renal disease Egyptian patients on maintenance hemodialysis.
Subjects
The present study included one hundred subjects, recruited from march to july 2014 from Medical Research Institute, divided into two main groups; the control group which included thirty apparently healthy subjectswith a median age 49 years, 14 (47.6%) were males and 16 (53.3%) females and the patients group which included seventy patients with end stage renal disease on maintenance haemodialysis with median 4 years. Their median age was 52 years, 33(47.1%) were males and 37 (52.9%)were females.
Methods
To all studied subjects, detailed history was taken, thorough physical examination, carotid intima media thickness (CIMT) [26], presence of plaques and ECG [27], ischemic changes. Laboratory investigations included serum levels [28] of: glucouse, urea, creatinine, uric acid, albumin, total cholesterol, low and high density lipoprotein- cholesterol, calcium, phosphorus, and CRP as well as plasma PTH level. For molecular studies [29,30], the detection of BsmI and FokI polymorphisms using polymerase chain reaction and restriction fragment length polymorphism (PCR / RFLP) technique. Statistical analysis [31], was done using SPSS program version 20 (Statistical Package of social sciences, Chicago, USA) [32], D’Agostino-Pearson K-squared test for normality ,Unpaired Student’s t-test, Mann Whitney test, The Chi-Square test (χ2 test), Fisher’s Exact Test waHardy-Weinberg Equilibrium using Pearson’s Chi-Squared goodness of fit test were used.
Results
For BsmI
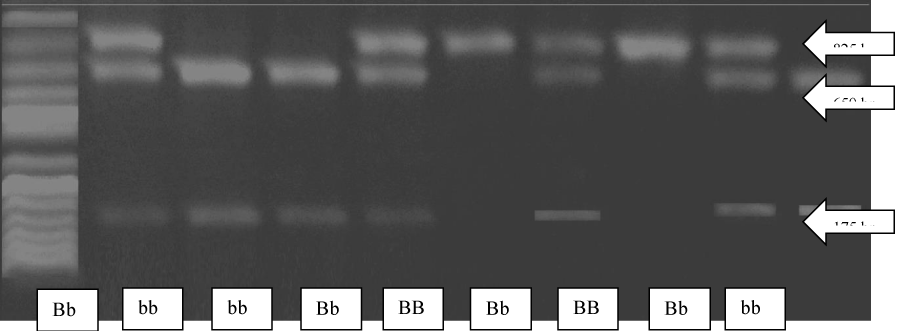
The 825 bp PCR product derived from an allele having a BsmI site in intron 8, designated b, where split into two bands: 650 bp and 175 bp upon digestion with BsmI. While those derived from an allele not having a BsmI site in the corresponding sequence, designated B, remained a single band with 825 bp. So there were three possible genotypes for BsmI gene polymorphism: BB (1 band at 825 bp), Bb (3 bands at 825,650 and 175 bp) and bb (2 bands at 650 and 175 bp) (Figure 1).
Figure 1: Agarose gel electrophoresis of PCR products digested with BsmI restriction enzyme visualized by ethidium bromide.
For FokI
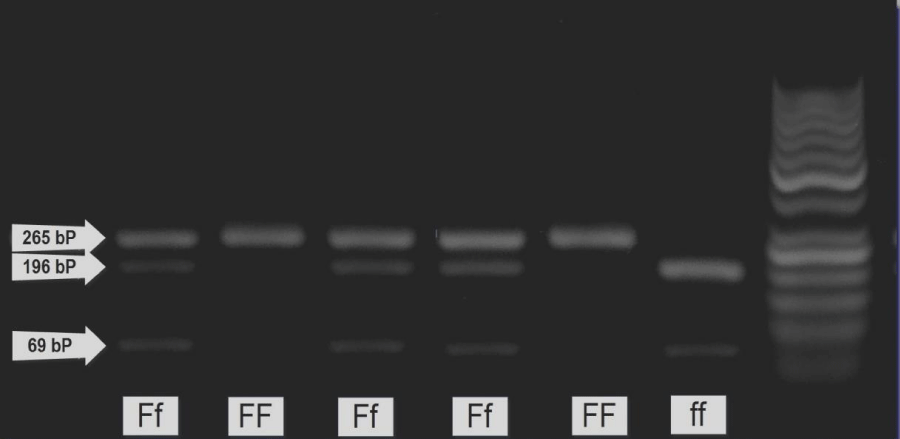
The 265 bp PCR product derived from an allele having a FokI site in exon 2, designated f, where split into two bands: 196 bp and 69 bp upon digestion with FokI. While those derived from an allele not having a FokI site in the corresponding sequence, designated F, remained a single band with 265 bp. So there were three possible genotypes for FokI gene polymorphism: FF (1 band at 265 bp), Ff (3 bands at 265,196 and 69 bp) and ff (2 bands at 196 and 69 bp) (Figure 2).
Figure 2: Agarose gel electrophoresis of PCR products digested with FokI restriction enzyme visualized by ethidium bromide.
Discussion
In end stage renal disease, the synthesis of vitamin D is disturbed [33]. Active vitamin D level decreases as renal function deteriorates and becomes very low or even undetectable. Low active vitamin D is well known to play an important role in the pathogenesis of hyperparathyroidism [34].
Hyperparathyroidism is one of the key factors in the pathogenesis of many of the complications of dialysis mainly bone and cardiovascular complications [35].
The VDR is an important element for physiologic regulation of parathyroid function and is responsible for the inhibitory effects of vitamin D on parathyroid hormone secretion and parathyroid cell proliferation [34].
Diminished activation of VDR in the parathyroid glands leads to increased release of PTH. Moreover, calcium sensing receptors (CaR) expressed in parathyroid glands react rapidly to extracellular calcium concentration and low calcium level leads to further increase in the release of PTH (secondary hyperparathyroidism) [34].
The present study aimed at assessing vitamin D receptor gene polymorphisms BsmIand FokI in Egyptian patients with end stage renal disease on maintenance haemodialysis. The assosciation of these polymorphisms with cardiovascular complications and hyperparathyroidism among these patients was also performed.
The present study included one hundred subjects, divided into two main groups. The control group included thirty apparently healthy volunteers; 14 males (46.7%) and 16 females (53.3%). Their age ranged from 42 to 69 years with median of 49 years. The patients group included seventy patients with end stage renal disease on maintenance haemodialysis; 33males (47.1%) and 37 females (52.9%).Their age ranged from 37 to 75 years with median of 52 years and their duration of dialysis ranged from 1-19 years with median of 4 years.
In the present work there was no significant difference between the control and total patients group regarding age and sex (P=0.36, 0.97 respectively) which denoted that both groups were of comparable conditions (Table 1).
| Table 1: Statistical analysis of some clinical data among the studied groups. | ||||
Range Median |
Control group (n=30) |
Total patients (n=70) |
P-value | |
| Age (years) |
Range | 42-69 | 37-75 | P=0.36 |
| Median | 49 | 52 | ||
| Sex | Females | 16 (53.3%) | 37 (52.9%) | P=0.97 |
| Males | 14 (46.7%) | 33 (47.1%) | ||
| Duration of dialysis (years) |
Range | - | 1-19 | |
| Median | - | 4 | ||
| SBP (mmHg) |
Range | 100-120 | 100-170 | P=0.000** |
| Median | 117.5 | 120 | ||
| DBP (mmHg) |
Range | 70-80 | 70-100 | P=0.000** |
| Median | 77.5 | 80 | ||
| MBP (mmHg) |
Range | 80-93.3 | 80-123.3 | P=0.000** |
| Median | 88.35 | 96.7 | ||
| CIMT (mm) |
0.29 | 0.704 | P=0.000** | |
| ± SD | 0.131 | 0.221 | ||
| Parametric data is presented by mean± standard deviation, Student t-test statistics for parametric data analysis, Nonparametric data is presented by median (Range) Mann-Whitney test statistics for non-parametric data analysis. SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, MBP: Mean Blood Pressure, CIMT: Carotid Intima Media Thickness. N: Number of subjects, ECG (+) = Electrocardiographic changes of ischemic heart diseases, Plaque (+) = presence of atherosclerotic plaque in the carotid arteries. n: Number of subjects. P: Statistical significance between control group and total patients group. *: Significant at P<0.05. **: Highly Significant at P<0.01. |
||||
In the current work, the distribution frequencies of BB, Bb, and bb genotypes in the control group were 23.3 %, 53.3 %, and 23.3 %; respectively. In the patients group, they were 28.6 %, 45.7 % and 25.7%; respectively (Table 2), showing no significant difference in the genotype distribution of BsmI polymorphism among the control group and the total patients group (X2 = 0.515 and p= 0.773). While, the distribution of B and b alleles were 50 % and 50 %; respectively in the control group and were 51.4 % and 48.6 % in the patients group (Table 3), showing no significant difference in the frequency of the BsmI alleles among the the control group and the total patients group (X2= 0.034, p= 0.853).
| Table 2: Genotype frequencies of BsmI polymorphism among the studied groups. | |||
| Genotype | n % | Control group (n=30) |
Total patients (n=70) |
| BB | n | 7 | 20 |
| % | 23.33 | 28.6 | |
| Bb | n | 16 | 32 |
| % | 53.33 | 45.7 | |
| bb | n | 7 | 18 |
| % | 23.33 | 25.7 | |
| Chi-square p-value |
0.515 0.773 |
||
| Table 3: BsmI allele frequency among the studied groups. | |||
| Allele | n % |
Control group (n=30) |
Total patients (n=70) |
| B | n | 30 | 72 |
| % | 50 | 51.4 | |
| b | n | 30 | 68 |
| % | 50 | 48.6 | |
| Chi-square p-value |
0.034 0.853 |
||
| n: Number of subjects. P<0.05 is significant | |||
In an Egyptian study conducted by El Gawad et al. (2012) [36], the distribution frequencies of BB, Bb, and bb genotypes in the control group were similar to the present study, showing 26.7 %, 50 %, and 23.3 % ; respectively. However, another study was conducted on Polish population by Kaleta et al. (2013) [10] and the genotype distribution among the control groups was as follows; 17% for the BB genotype, 42% for the Bb genotype and 41% for the bb genotype. This difference could be attributed to racial variability in the studied populations.
In addition, the genotype distribution of the BsmI polymorphism in the present study did not deviate from the Hardy-Weinberg equilibrium (HWE) among the control group and among the total patients group (Table 4a: X2 = 0.133, 0.505 and p= 0.715, 0.477; respectively).
| Table 4a: Comparison between BsmI polymorphism genotype frequencies among the studied groups with Hardy-Weinberg (HW) prediction using Chi-square (X2) analysis. | ||||
| Genotype | Count HW prediction |
Control group (n=30) |
Total patients (n=70) |
Total subjects (n=100) |
| BB | Observed | 7 | 20 | 27 |
| HW Expected | 7.5 | 18.5 | 26 | |
| Bb | Observed | 16 | 32 | 48 |
| HW Expected | 15 | 35 | 50 | |
| bb | Observed | 7 | 18 | 25 |
| HW Expected | 7.5 | 16.5 | 24 | |
| X2 p-value |
0.133 0.715 |
0.505 0.477 |
0.157 0.692 |
|
| n: Number of subjects. P<0.05 is significant |
||||
As regards FokI in the current study, the distribution frequencies of FF, Ff, and ff genotypes in the control group were 56.7 %, 40 %, and 3.3 %; respectively. In the patients group, they were 57.1 %, 31.4 % and 11.4 %; respectively (Table 4b). There was no significant difference in the genotype distribution of FokI polymorphism among the the control group and the total patients group (X2 = 1.984 and p=0.371).
| Table 4b: Genotype frequencies of FokI polymorphism among the studied groups. | |||
| Genotype | n % |
Control group (n=30) |
Total patients (n=70) |
| FF | n | 17 | 40 |
| % | 56.7 | 57.1 | |
| Ff | n | 12 | 22 |
| % | 40 | 31.4 | |
| ff | n | 1 | 8 |
| % | 3.3 | 11.4 | |
| Chi-square p-value |
1.984 0.371 |
||
Similar results were obtained in the study of Vigo et al. (2005) [25], which was conducted on Spanish patients with chronic renal failure, the FokI genotype frequencies in the healthy controls were 46.7% FF, 43.3% Ff and 10% ff vs 54.7% FF, 28.1% Ff and 17.2% ff in the CRF patients. Another study was conducted on Polish population by Mostowska et al. (2013) [37] and the genotype distribution among the control groups was as follows; 37% for the FF genotype, 45% for the Ff genotype and 18% for the ff genotype.
In addition, the present study showed that the distribution of F and f alleles were 76.7% and 23.3 %; respectively in the control group and 72.9 % and 27.1 % in the patients group (Table 4c). There was no significant difference in the frequency of the FokI alleles among the studied groups (X2= 0.317, p= 0.574).
| Table 4c: FokI allele frequency among the studied groups. | |||
| Allele | n % | Control Group (n=30) |
Total patients (n=70) |
| F | n | 46 | 102 |
| % | 76.7 | 72.9 | |
| f | n | 14 | 38 |
| % | 23.3 | 27.1 | |
| Chi-square p-value |
0.317 0.574 |
||
| n: Number of subjects, P<0.05 is significant. | |||
In the present study, the genotype distribution patterns of FokI polymorphism among the control group and among the total patients group were also found to be in agreement with HWE (Table 4d : X2 = 0.418, 2.952 and p= 0.518, 0.0857; respectively).
| Table 4d: Comparison between FokI polymorphism genotype frequencies among the studied groups with Hardy-Weinberg (HW) prediction using Chi-square (X2) analysis. | ||||
| Genotype | Count HW prediction |
Control group (n=30) | Total patients (n=70) | Total subjects (n=100) |
| FF | Observed | 17 | 40 | 57 |
| HW Expected | 17.6 | 37.2 | 54.8 | |
| Ff | Observed | 12 | 22 | 34 |
| HW Expected | 10.7 | 27.7 | 38.5 | |
| ff | Observed | 1 | 8 | 9 |
| HW Expected | 1.6 | 5.2 | 6.8 | |
| X2 p-value |
0.418 0.518 |
2.952 0.0857 |
1.355 0.244 |
|
| n: Number of subjects. P<0.05 is significant |
||||
Assessment of atherosclerosis was achieved in this study by carotid ultrasonography [11]. There was a significant increase in CIMT in the total patients group than in the controls (P= 0.000) (Table 1). The present result was in agreement with the recent study of Paul et al. (2012) [38, who found that patients on haemodialysis had significantly greater CIMT than age- and sex-matched non-dialyzed chronic renal failure patients, suggesting that hemodialysis is an independent risk factor for atherosclerosis in CRF patients.
In the present work, there were 37 patients (52.9% of total patients) with high CIMT values (whom were chosen as having CIMT > 0.6 mm which was the highest value in the control group). While 33 patients (47.1%) had CIMT ≤ 0.6 mm (Table 4e). There was no significant difference in the genotype distribution of both BsmI and FokI polymorphisms among the patients with high and normal CIMT (X2 = 0.19, 1.78 and p= 0.91, 0.41; respectively)
| Tables 4e: Frequency of patients with ESRD on maintenance hemodialysis with cardiovascular complications and hyperparathyroidism. | ||||
| High CIMT (mm) |
Plaque (+) | ECG (+) | Hyperparathyroidism (PTH > 300 pg/mL ) |
|
| Number | 37 | 27 | 36 | 43 |
| % of patients | 52.9 | 38.6 | 51.4 | 61.4 |
| CIMT: Carotid intima media thickness Plaque (+)= presence of atherosclerotic plaque in the carotid arteries ECG (+)= Electrocardiographic changes of ischemic heart diseases PTH: Parathyroid hormone - Patients with high CIMT were chosen as patients having CIMT > 0.6 mm which was the highest value in the control group. - Hyperparathyroid patients were chosen as patients having PTH level > 300 pg/mL. |
||||
Carotid ultrasonography also allows visualization and measurement of the extent of carotid plaques which are considered a direct reflection of the atherosclerotic process [12]. In the present study, carotid plaques were present in 27 patients (38.6% of total patients), while 43 patients (61.4% total patients) did not had carotid plaques. (Table 4e) There was no significant difference in the genotype distribution of both BsmI and FokI polymorphisms among the patients with and without carotid plaques (X2 = 0.94, 2.19 and p= 0.63, 0.33; respectively).
In addition, blood pressure (systolic, diastolic, and mean blood pressure) was significantly higher in total patients group than in control group [P=0.000 (SBP), P=0.000 (DBP), P=0.000 (MBP)] (Table 1). It is known that hypertension is a common finding in dialysis patients, and based upon multiple studies, over 50 to 60 percent of hemodialysis patients (up to 85 percent in some reports) are hypertensive [39,40]. It is known that the etiology of hypertension in end-stage renal disease is multifactorial which may be due to sodium and volume excess due to diminished sodium excretory capacity, activation of the renin-angiotensin-aldosterone system, increased activity of the sympathetic nervous system, administration of erythropoietin, calcification of the arterial tree, and preexistent essential hypertension [41,42].
In the present work, the ischemic ECG changes were present in 36 patients (51.4% of total patients), while 34 patients (48.6% of total patients) did not have ECG changes. There was no significant difference in the genotype distribution of both BsmI and FokI polymorphisms among the patients with and without ECG changes (X2 = 0.068, 2.126 and p= 0.97, 0.35; respectively).
In the present study, the patients group was further subdivided into two subgroups according to the presence and abscence of cardiovascular complications as evidenced by the presence of one or more of the following: high carotid intima media thickness measurement (CIMT > 0.6 mm) or presence of plaques or presence of ECG changes. Group A included 30 patients without cardiovascular complications and Group B included 40 patients with cardiovascular complications.
The distribution frequencies of BB, Bb, and bb genotypes were 30 %, 43.3 %, and 26.7 %; respectively in the patients without cardiovascular complications (group A) and 27.5 %, 47.5 % and 25 %; respectively in patients with cardiovascular complications (group B) (Table 5a). No significant difference was observed in the genotype distribution of BsmI polymorphism among patients with and without cardiovascular complications in the patients group (X2 = 0.121 and p= 0.941).
| Table 5a: Genotype frequencies of BsmI polymorphism among patients with and without cardiovascular complications in the patients group with ESRD on maintenance haemodialysis: | |||
| Genotype | n % |
Patients without cardiovascular complications (group A) (n=30) |
Patients with cardiovascular complications (group B) (n=40) |
| BB | n | 9 | 11 |
| % | 30 | 27.5 | |
| Bb | n | 13 | 19 |
| % | 43.3 | 47.5 | |
| bb | n | 8 | 10 |
| % | 26.7 | 25 | |
| Chi-square p-value |
0.121 0.941 |
||
| n: Number of subjects, P<0.05 is significant | |||
In addition, no significant difference was observed in the genotype distribution of FokI polymorphism among patients with and without cardiovascular complications in the patients group (X2 = 1.502 and p= 0.472). The distribution frequencies of FF, Ff, and ff genotypes were 56.67 %, 36.67 %, and 6.67 %; respectively in patients group A, while 57.5 %, 27.5 % and 15 %; respectively in patients group B (Table 5b).
| Table 5b: Genotype frequencies of FokI polymorphism among patients with and without cardiovascular complications in the patients group with ESRD on maintenance haemodialysis: | |||
| Genotype | n % |
Patients without cardiovascular complications (group A) (n=30) |
Patients with cardiovascular complications (group B) (n=40) |
| FF | n | 17 | 23 |
| % | 56.67 | 57.5 | |
| Ff | n | 11 | 11 |
| % | 36.67 | 27.5 | |
| ff | n | 2 | 6 |
| % | 6.67 | 15 | |
| Chi-square p-value |
1.502 0.472 |
||
| n: Number of subjects, P<0.05 is significant | |||
The present results were in agreement with that of Pan et al. (2009) [43], in which no significant differences were observed in the genotype and allele frequencies of both BsmI and FokI polymorphisms between the two groups. Another study by Ortlepp et al. (2003) [44], found that the VDR gene variant BsmI was not associated with prevalence and severity of coronary artery disease in a large-scale cohort phenotyped by angiography. In addition, there is an agreement with Yokoyama et al. (2012) [45], who found that FokI polymorphisms by themselves showed no significant associations with CKD stage.
C-reactive protein was significantly higher in total patients group than control group (P=0.000) (Table 4e). CRP is an active participant in pro-atherosclerotic phenomenon including local pro-inflammatory and thrombotic events. Baravkar et al. (2013) [46], concluded that CRP estimation in CKD is helpful in predicting an increased risk of cardiovascular death.
In the current work, serum calcium and corrected calcium were significantly lower in total patients group than control group (P=0.000). Phosphorus and Ca-P product were significantly higher in total patients group than control group (P=0.000). In addition, there was a significant increase in the parathyroid hormone levels in total patients group than control group (P=0.000) (Table 5c). Secondary hyperparathyroidism is a common occurrence in patients with chronic renal failure and is characterized by excessive serum parathyroid hormone levels, parathyroid hyperplasia and imbalances in calcium and phosphorus metabolism [47].
| Table 5c: Statistical analysis of the serum levels of some studied parameters among the studied groups. | ||||
Range Median |
Control group (n=30) | Total patients (n=70) | P-value | |
| FSG (mg/dl) | Range | 82-104 | 72-546 | P=0.000** |
| Median | 94 | 105 | ||
| Urea (mg/dl) | 33.8 | 155.8 | P=0.000** | |
| ± SD | 5.4 | 36.19 | ||
| Creatinine (mg/dl) | 0.90 | 10.2 | P=0.000** | |
| ± SD | 0.14 | 2.82 | ||
| Uric acid (mg/dl) | 4.7 | 6.52 | P=0.000** | |
| ± SD | 0.95 | 1.24 | ||
| Albumin (g/dl) | Range | 3.9-4.7 | 3-5 | P=0.000** |
| Median | 4.12 | 3.9 | ||
| TG (mg/dl) |
Mean | 108.3 | 175.1 | P=0.000** |
| ± SD | 24.41 | 81.57 | ||
| TC (mg/dl) |
Mean | 171.4 | 181.9 | P=0.176 |
| ± SD | 22.68 | 53.93 | ||
| HDL-C (mg/dl) |
Mean | 52.9 | 35.0 | P=0.000** |
| ± SD | 6.46 | 7.56 | ||
| LDL-C (mg/dl) |
Mean | 96.9 | 110.5 | P=0.04* |
| ± SD | 21.87 | 43.08 | ||
| CRP (mg/L) |
Mean | 1.1 | 6.2 | P=0.000** |
| ± SD | 0.36 | 4.1 | ||
| Calcium (mg/dl) |
Mean | 9.5 | 8.65 | P=0.000** |
| ± SD | 0.25 | 0.82 | ||
| corrected calcium (mg/dl) |
Mean | 9.38 | 8.73 | P=0.000** |
| ± SD | 0.24 | 0.84 | ||
| Phosphorus (mg/dl) |
Mean | 3.51 | 5.83 | P=0.000** |
| ± SD | 0.28 | 1.34 | ||
| Ca-P product (mg2/dl2) |
Mean | 32.86 | 50.88 | P=0.000** |
| ± SD | 2.55 | 12.93 | ||
| PTH ( pg/ml ) |
Range | 17.6-27.8 | 39.6-2786.4 | P=0.000** |
| Median | 21.65 | 380.6 | ||
| Parametric data is presented by mean± standard deviation Student t-test statistics for parametric data analysis Nonparametric data is presented by median (Range) Mann-Whitney test statistics for non-parametric data analysis. n: Number of subjects. P: Statistical significance between control group and total patients group. *: Significant at P<0.05. **: Highly Significant at P<0.01. |
||||
The patient group was further subdivided into two subgroups according to the development of hyperparathyroidism as evidenced by PTH level. Group C included 27 patients with PTH level < 300 pg/mL and and Group D included 43 patients with PTH level > 300 pg/mL.
The distribution frequencies of BB, Bb, and bb genotypes were 33.3%, 51.9%, and 14.8%; respectively in patients with PTH level < 300 pg/mL (group C), while 25.6%, 41.9% and 32.6%; respectively in patients with PTH level > 300 pg/mL (group D). No significant difference was observed in the genotype distribution of BsmI polymorphism among group C and group D patients (X2 = 2.742 and p= 0.254) (Table 5d).
| Table 5d: Genotype frequencies of BsmI polymorphism among patients with PTH level < 300 pg/mL and patients with PTH level > 300 pg/mL in the patients group with ESRD on maintenance haemodialysis: | |||
| Genotype | n % |
Patients with PTH level < 300 pg/mL (group C) (n=27) |
Patients with PTH level > 300 pg/mL (group D) (n=43) |
| BB | n | 9 | 11 |
| % | 33.3 | 25.6 | |
| Bb | n | 14 | 18 |
| % | 51.9 | 41.9 | |
| bb | n | 4 | 14 |
| % | 14.8 | 32.6 | |
| Chi-square p-value |
2.742 0.254 |
||
| n: Number of subjects, P<0.05 is significant | |||
The current work agrees with Ozdemir et al. (2005) [35], who found no association between secondary hyperparathyroidism and BsmI variants in Turkish end stage renal patients. They found that the BB genotype and B allele frequencies were not statistically different in the hypo- and hyperparathyroid patients.
Conversely, Fernandez et al. [23], reported that the BB genotype and the B allele were significantly more frequent in Spanish patients with end-stage renal disease with low iPTH levels than in patients with high iPTH levels (32.3% versus 12.5% and 58.8% versus 39.1%; respectively).
Similarly, Tagliabue et al. [48], reported a higher frequency of BB variant among Italian dialysis patients with hypoparathyroidism compared to patients with secondary hyperparathyroidism (34% versus 16%). Also, a protective effect of the B allele has been reported in Japanese patients undergoing hemodialysis [49].
The discovery of the VDR on parathyroid cells opened an exciting field of research since mid-nineties. In 1995 Carling et al. [50], reported a relationship between BsmI polymorphism and primary HPT. These results led to the hypothesis that VDR polymorphisms could be involved in sHPT due to CRF. Tsukamoto et al. [51], reported a higher incidence of the b allele on hemodialysis patients with sHPT.
Valdivielso (2006) [52], designed a bigger study minimizing the impact of the time on hemodialysis as an important risk factor for the development of sHPT.
It is very difficult to determine the increase in the risk of developing sHPT associated with having a specific BsmI genotype. Firstly, because of the different and, sometimes, contradictory results found in the literature. Secondly, due to the fact that the risk calculation is performed with many variables, and some of those change within different studies (sample size, study design, etc.). However, almost all the reports published indicate that there is some influence of BsmI genotype on the sHPT progress. In an ideal scenario to prevent sHPT in early stages of CRF, the use of BsmI polymorphism to evaluate the risk of the patients to develop sHPT could be an option [5].
The association of BsmI genotype with PTH levels has also been tested after kidney transplantation. In this case, all the reports published so far agree in the association of the bb genotype with higher PTH levels [53,54].
Also in the present study, no significant difference was observed in the genotype distribution of FokI polymorphism among patients with PTH level < 300 pg/mL (group C) and patients with PTH level > 300 pg/mL (group D) (X2 = 0.074 and p= 0.964). The distribution frequencies of FF, Ff, and ff genotypes were 55.6 %, 33.3 %, and 11.1 %; respectively in the patients group C, while 58.1 %, 30.2 % and 11.6 %; respectively in the patients group D (Table 5e).
| Table 5e: Genotype frequencies of FokI polymorphism among patients with PTH level < 300 pg/mL and patients with PTH level > 300 pg/mL in the patients group with ESRD on maintenance haemodialysis: | |||
| Genotype | n % |
Patients with PTH level < 300 pg/mL (group C) (n=27) |
Patients with PTH level > 300 pg/mL (group D) (n=43) |
| FF | N | 15 | 25 |
| % | 55.6 | 58.1 | |
| Ff | N | 9 | 13 |
| % | 33.3 | 30.2 | |
| ff | n | 3 | 5 |
| % | 11.1 | 11.6 | |
| Chi-square p-value |
0.074 0.964 |
||
| n: Number of subjects, P<0.05 is significant | |||
In the study of Vigo et al. (2005) [25], they found that within the CRF patients, the mean serum PTH level in the FF group was significantly higher (159.77+/-25.69 pg/ml) than in both the Ff and ff groups (106.67+/-19.07 and 77.55+/-15.85 pg/ml, respectively; p<0.05). These results suggest that FokI polymorphisms of the VDR gene may determine parathyroid response in CRF patients. Due to the higher sensitivity of the FF phenotype to 1, 25(OH)2D3, it would be reasonable to expect the opposite result. However, the authors argue that, before the onset of renal failure, the patients with the FF phenotype had a stronger suppression of the PTH levels. Thus, after the renal failure and the decrease of 1, 25(OH)2D3 levels, the lack of constrain on PTH secretion would lead to higher increases on PTH levels in the FF patients. In predialysis patients, levels of 1, 25(OH)2D3 are low. Thus, the authors argue that the higher sensitivity of the FF genotype will be suppressing the PTH levels on [25].
From the genetic perspective, it is important to note that the FokI RFLP can be considered an independent marker in the VDR gene since there is no linkage disequilibrium (LD) with any of the other VDR polymorphisms and the LD area surrounding this polymorphism seems to be very small [55].
Differences in race, diet or even latitude could alter the influence of the polymorphisms on the susceptibility to diseases, diluting the effects observed in other populations [52]. The role of VDR polymorphisms will still be a topic for discussion.
From the present study the following could be concluded
1. No statistically significant difference could be detected in both BsmI and FokI gene polymorphisms between the hemodialysis patients and the controls, suggesting that the development of ESRD had no relation with either VDR BsmI or FokI gene polymorphisms.
2. No statistically significant difference were found in these polymorphisms between the hemodialysis patients with or without cardiovascular complications or between patients with PTH level less or more than 300 pg/ml. These results suggest that the development of cardiovascular complications and secondary hyperparathyroidism among Egyptian patients on maintenance haemodialysis cannot be attributed to these two gene polymorphisms.
3. No association could be found between the variant alleles of BsmI and FokI gene polymorphisms and the development of ESRD, cardiovascular complications and secondary hyperparathyroidism among the studied samples of Egyptian patients on maintenance haemodialysis.
Recommendations
1. It is recommended to carry out further studies on BsmI and FokI gene polymorphisms in larger groups of haemodialysis patients.
2. Further studies are needed on other VDR polymorphisms and their assosciation with ESRD and its complications.
Limitation of the study
The use of VDR polymorphisms as diagnostic tools, or even as markers for a higher propensity to suffer some diseases, is still a matter of debate.
Further effort must be placed in understanding the molecular and cellular variations affected by the polymorphisms and in performing observational studies in bigger populations.
Special attention should be paid to the effects of environmental contributions on VDR polymorphisms in various populations.
References
- Tang J. Vitamin D and its role in chronic kidney disease. Nephrology Rounds. 2009; 7: 1-6.
- Feldman D, Pike JW, Adams JS. Vitamin D, 3rd Ed. Academic Press Elsevier, Amsterdam. 2011; 1245-90.
- Hollick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266-2681. Ref.: https://goo.gl/6ioGRP
- Grzegorzewska AE, Świderska MK, Mostowska A, Warchoł W, Jagodziński PP. Polymorphisms of vitamin D receptorsignaling pathway genes and calcium-sensing receptor gene in respect to survival of hemodialysis patients: A prospective observational study. Int J Endocrinol. 2016; 2383216: 11. Ref.: https://goo.gl/Vm7YAc
- Kato S. The function of vitamin D receptor in vitamin D action. J Biochem. 2000; 127: 717-722. Ref.: https://goo.gl/2UKFNz
- Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008; 67: 115-127. Ref.: https://goo.gl/PNpRJL
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004; 80: 1678-1688. Ref.: https://goo.gl/MDQHxA
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006; 58: 685-704. Ref.: https://goo.gl/jQsXra
- Genetics home reference. http://ghr.nlm.nih.gov/gene/VDR.
- Bouillon R, Carmeliet G, Verlinden L, Van Etten E, Verstuyf A, et al. Vitamin D and Human Health: Lessons from vitamin D receptornull mice. Endocr Rev. 2008; 29: 726-776. Ref.: https://goo.gl/mZMxYS
- Valdivielso JM, Fernandez E. Vitamin D receptor polymorphism and disease. Clin Chim Acta. 2006; 371: 1-12. Ref.: https://goo.gl/4Tvt1s
- Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L. Natural selection has driven population differentiation in modern humans. Nat Genet. 2008; 40: 340-345. Ref.: https://goo.gl/6V16kH
- Bid HK, Mittal RD. Study of vitamin-D receptor (VDR) gene start codon polymorphism (Fok I) in healthy individuals from North India. Indian J Hum Genet. 2003; 9: 51-4.
- Li WH, Gu Z, Wang H, Nekrutenko A. Evolutionary analyses of the human genome. Nature 2001; 409: 847-849. Ref.: https://goo.gl/jE2BEY
- Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004; 338: 143-156. Ref.: https://goo.gl/mtd2hs
- Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000; 22: 203-217. Ref.: https://goo.gl/nRPwNd
- http://www.snpedia.com/index.php/Rs1544410
- Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, et al. Functionally relevant polymorphisms in the human nuclear vitamin Dreceptor gene. Mol Cell Endocrinol. 2001; 177: 145-1459. Ref.: https://goo.gl/VdS6vT
- http://www.snpedia.com/index.php/Rs2228570
- Mosos I, Marginean O. Links between vitamin Ddeficiency and cardiovascular diseases. Biomed Res Int. 2015; 109275: 12. Ref.: https://goo.gl/9puMuk
- Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease-mineral bone disease (CKD-MBD). Bonekey Rep. 2014; 3: 498. Ref.: https://goo.gl/853Fi
- Tomasello S. Secondary hyperparathyroidism and chronic kidney disease. Diabetes Spectrum 2008; 21: 19-25.
- Santoro D, Lucisano S, Gagliostro G, Alibrandi A, Benvenga S, et al. Vitamin D receptor polymorphism in chronic kidney disease patients with complicated cardiovascular disease. J Ren Nutr. 2015; 25: 187-193. Ref.: https://goo.gl/JDdtPo
- Fernandez Fernández E, Fibla J, Betriu A, Piulats JM, Almirall J, et al. Association between vitamin D receptor gene polymorphism and relative hypoparathyroidism in patients with chronic renal failure. J Am Soc Nephrol. 1997; 8: 1546-1552. Ref.: https://goo.gl/LGJ3XU
- Vigo GE, Cadarso-Suarez C, Perez-Fernandez R, Romero BR, Devesa MJ, et al. Association between vitamin D receptor FokI Polymorphism and serum parathyroid hormone level in patients with chronic renal failure. J Endocrinol Invest. 2005; 28: 117-121. Ref.: https://goo.gl/nSpKAD
- Yildiz A, Tepe S, Olfaz H, Yazici H, Ark E, et al. Carotid atherosclerosis is a predictor of coronary calcification in chronic hemodialysis patients. Nephrol Dial Transplant. 2004; 19: 885-891. Ref.: https://goo.gl/cQV5ic
- Ruiz MR, Cajavilca C, Varon J. Einthoven's string galvanometer the first electrocardiograph. Tex Heart Inst J. 2008; 35: 174-178. Ref.: https://goo.gl/QpEFcC
- Burtis CA, Ashwood ER, Bruns DE. Tietz Text Book of Clinical Chemistry and Molecular Diagnostics. 4th Ed. Elsevier Saunders Company, St Louis. 2006; 1689-1699.
- Gunes S, Sumer AP, Keles GC, Kara N, Koprulu H, et al. Analysis of vitamin D receptor gene polymorphisms in patients with chronic periodontitis. Indian J Med Res. 2008; 127: 58-64. Ref.: https://goo.gl/RUuhpf
- Mansour L, Sedky M, AbdelKhader M, Sabry R, Kamal M, et al. The role of vitamin D receptor genes (FOKI and BSMI) polymorphism in osteoporosis. Middle East Fertil Soc J. 2010; 15: 79-83.
- Daly LE, Bourke GJ. Interpretation and uses of medical statistics. 5th Ed. Blackwell Science, Oxford, Malden. 2000.
- Puri BK. SPSS in practice: an illustrated guide. 2nd Ed. Arnold, London, New York. 2002.
- Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006; 65: 33-43. Ref.: https://goo.gl/qJpaVV
- Kumar P, Clark M. Kumar and Clark's Clinical Medicine. 7th Ed. Elsevier Saunders Company, Edinburgh, 2009; 628.
- Ozdemir FN, Sezer S, Atac B, Tutal E, Verdi H, et al. Vitamin D receptor BsmI and TagI gene polymorphisms in a Turkish ESRD population: influences on parathyroid hormone response. Transplant Proc. 2005; 37: 2922-2924. Ref.: https://goo.gl/JPJepx
- El Gawad SS, Samea ER, Sherif M, Elrakhawy MM. Vitamin D receptor genotypes, bone mineral density and biochemical markers of bone turnover in Egyptian premenopausal women with graves ' disease. New York Science Journal. 2012; 5: 54-61.
- Mostowska A, Lianeri M, Wudarski W, Olesińska M, Jagodziński PP. Vitamin D receptor gene BsmI, FokI, ApaI and TaqI polymorphisms and the risk of systemic lupus erythematosus. Mol Biol Rep. 2013; 40: 803-810. Ref.: https://goo.gl/EQBP9w
- Paul J, Dasgupta S, Ghosh MK. Carotid artery intima media thickness as a surrogate marker of atherosclerosis in patient with chronic renal failure on hemodialysis. N Am J Med Sci. 2012; 4: 77-80. Ref.: https://goo.gl/xBx8HS
- Rahman M, Smith MC. Hypertension in hemodialysis patients. Curr Hypertens Rep. 2001; 3: 496-502. Ref.: https://goo.gl/4FHxRf
- Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, et al. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003; 115: 291-297. Ref.: https://goo.gl/BoHgR3
- Agarwal R. Hypertension and survival in chronic hemodialysis patients-past lessons and future opportunities. Kidney Int. 2005; 67: 1-13. Ref.: https://goo.gl/ZoNdVG
- Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, et al. Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 2006; 17: 513-20. Ref.: https://goo.gl/ADveVN
- Pan XM, Li DR, Yang L, Wang EY, Chen TY, et al. No association between vitamin D receptor polymorphisms and coronary artery disease in a Chinese population. DNA Cell Biol 2009; 28: 521-525. Ref.: https://goo.gl/AE6sKT
- Ortlepp JR, Von Korff A, Hanrath P, Zerres K, Hoffmann R. Vitamin D receptor gene polymorphism BsmI is not associated with the prevalence and severity of CAD in a large-scale angiographic cohort of 3441 patients. Eur J Clin Invest. 2003; 33: 106-109. Ref.: https://goo.gl/EdWStv
- Yokoyama K, Nakashima A, Urashima M, Suga H, Mimura T, et al. Interactions between serum vitamin D levels and vitamin D receptor gene FokI polymorphisms for renal function in patients with type 2 Diabetes. PLoS One. 2012; 7: 16. Ref.: https://goo.gl/DHrnqj
- Baravkar PN, Bavikar JS, Asegaonkar SB, Bavikar SS, Bardapurkar JS, et al. Study of serum uric acid and C-reactive protein levels in patients with chronic renal disease. Int J Biol Med Res. 2013; 4: 2758-2761.
- Horl WH. The clinical consequences of secondary hyperparathyroidism: focus on clinical outcomes. Nephrol Dial Transplant. 2004; 19: 2-8. Ref.: https://goo.gl/k7FkdF
- Tagliabue J, Farina M, Imbasciati E, Vergani C, Annoni G. BsmI polymorphism of the vitamin D receptor gene in hyperparathyroid or hypoparathyroid dialysis patients. Am J Clin Pathol. 1999; 112: 366-370. Ref.: https://goo.gl/q9eGr3
- Nagaba Y, Heishi M, Tazawa H, Tsukamoto Y, Kobayashi Y. Vitamin D receptor gene polymorphisms affect secondary hyperparathyroidism in hemodialyzed patients. Am J Kidney Dis 1998; 32: 464-469. Ref.: https://goo.gl/eLjFSy
- Carling T, Kindmark A, Hellman P, Lundgren E, Ljunghall S, et al. Vitamin-D-receptor genotypes in primary hyperparathyroidism. Nat Med 1995; 1: 1309-1311. Ref.: https://goo.gl/C83PL2
- Tsukamoto Y, Heishi M, Nagaba Y, Kobayashi N, Nomura Y, et al. More on hyperparathyroidism and the vitamin D receptor. Nat Med. 1996; 2: 1162. Ref.: https://goo.gl/MXmztE
- Valdivielso JM, Fernandez E. Vitamin D receptor polymorphism and disease. Clin Chim Acta. 2006; 371: 1-12. Ref.: https://goo.gl/6nh7yN
- Giannini S, D'Angelo A, Nobile M, Carraro G, Rigotti P, et al. The effects of vitamin D receptor polymorphism on secondary hyperparathyroidism and bone density after renal transplantation. J Bone Miner Res. 2002; 17: 1768-1773. Ref.: https://goo.gl/vjM6Lz
- Falkiewicz K, Bidzinska B, Demissie M, Boratyńska M, Zmonarski SC, et al. Influence of vitamin D receptor gene polymorphisms on secondary hyperparathyroidism and bone density after kidney transplantation. Transplant Proc. 2005; 37: 1023-1025. Ref.: https://goo.gl/TXpce2
- Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004; 338: 143-1456. Ref.: https://goo.gl/wYBjkt