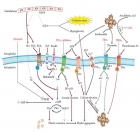
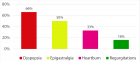
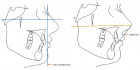
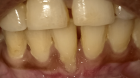
Abstract
Published: 01 January, 1970 | Volume - Issue | Pages:
Read Full Article HTML DOI: Cite this Article Read Full Article PDF
References
Similar Articles
-
The Risk-Adjusted Impact of Intraoperative Hemofiltration on Real-World Outcomes of Patients Undergoing Cardiac SurgeryMatata BM*,Shaw M. The Risk-Adjusted Impact of Intraoperative Hemofiltration on Real-World Outcomes of Patients Undergoing Cardiac Surgery. . 2017 doi: 10.29328/journal.jcn.1001001; 1: 001-010
-
Cardiac Manifestations on Anti-Phospholipid SyndromeFaisal AH*. Cardiac Manifestations on Anti-Phospholipid Syndrome. . 2017 doi: 10.29328/journal.jcn.1001002; 1: 011-013
-
Intraperitoneal and Subsequent Intravenous Vancomycin: An Effective Treatment Option for Gram-Positive Peritonitis in Peritoneal DialysisBarone RJ*,Gimenez NS,Ramirez L. Intraperitoneal and Subsequent Intravenous Vancomycin: An Effective Treatment Option for Gram-Positive Peritonitis in Peritoneal Dialysis. . 2017 doi: 10.29328/journal.jcn.1001003; 1: 014-018
-
Acute Tubulointerstitial Nephritis due to Phenytoin: Case ReportNilzete Liberato Bresolin*,Pedro Docusse Junior,Maria Beatriz Cacese Shiozawa,Marina Ratier de Brito Moreira,Natalia Galbiatti Silveira. Acute Tubulointerstitial Nephritis due to Phenytoin: Case Report. . 2017 doi: 10.29328/journal.jcn.1001004; 1: 019-025
-
Profile of vitamin D receptor polymorphism Bsm I and FokI in end stage renal disease Egyptian patients on maintenance hemodialysisEL-Attar HA*,Mokhtar MM,Gaber EW. Profile of vitamin D receptor polymorphism Bsm I and FokI in end stage renal disease Egyptian patients on maintenance hemodialysis. . 2017 doi: 10.29328/journal.jcn.1001005; 1: 026-040
-
Anemia response to Methoxy Polyethylene Glycol-Epoetin Beta (Mircera) versus Epoetin Alfa (Eprex) in patients with chronic Kidney disease on HemodialysisAlaa K Dhayef*,Jawad K Manuti,Abdulwahab S Abutabiekh. Anemia response to Methoxy Polyethylene Glycol-Epoetin Beta (Mircera) versus Epoetin Alfa (Eprex) in patients with chronic Kidney disease on Hemodialysis. . 2017 doi: 10.29328/journal.jcn.1001006; 1: 041-047
-
The outcome of Acute Kidney Injury in patients with severe MalariaJoão Egidio Romão Jr*,João Alberto Brandão. The outcome of Acute Kidney Injury in patients with severe Malaria. . 2017 doi: 10.29328/journal.jcn.1001007; 1: 048-054
-
Short term effect of Intravenous Intermittent Iron Infusion versus Bolus Iron Infusion on Iron parameters in Hemodialysis patientsIman Ibrahim Sarhan,Hussein Sayed Hussein,Islam Omar Elshazly*,Mahmoud Salah Hassan. Short term effect of Intravenous Intermittent Iron Infusion versus Bolus Iron Infusion on Iron parameters in Hemodialysis patients. . 2017 doi: 10.29328/journal.jcn.1001008; 1: 055-059
-
Association between bh4/bh2 ratio and Albuminuria in Hypertensive Type -2 Diabetic patientsJose Aviles-Herrera,Karla C Arana-Pazos,Leonardo Del Valle-Mondragon,Carolina Guerrero-García,Alberto Francisco Rubio-Guerra*. Association between bh4/bh2 ratio and Albuminuria in Hypertensive Type -2 Diabetic patients. . 2017 doi: 10.29328/journal.jcn.1001009; 1: 060-063
-
Posterior Reversible Leukoencephalopathy Syndrome in a patient after second dose of Rituximab for treatment of resistant Thrombotic Thrombocytopenic PurpuraSabaa Asif*,Sumbal Nasir Mahmood,Osama Kunwer Naveed. Posterior Reversible Leukoencephalopathy Syndrome in a patient after second dose of Rituximab for treatment of resistant Thrombotic Thrombocytopenic Purpura . . 2018 doi: 10.29328/journal.jcn.1001010; 2: 001-004
Recently Viewed
-
Investigate the Effect of Coating Concentration and Coating Thickness on the Anti-microbial Properties of Polycarbonate SheetSaleh Alkarri. Investigate the Effect of Coating Concentration and Coating Thickness on the Anti-microbial Properties of Polycarbonate Sheet. Ann Biomed Sci Eng. 2024: doi: 10.29328/journal.abse.1001029; 8: 011-020
-
Emerging Risk of Microplastics on Health, Agriculture and EnvironmentShreetam Parida, Nivethitha Ashok and Rajendra Kurapati*. Emerging Risk of Microplastics on Health, Agriculture and Environment. Ann Biomed Sci Eng. 2024: doi: 10.29328/journal.abse.1001028; 8: 004-010
-
Elderly (> 70 years) Multiple Myeloma Patients Benefit Equally from Autologous Hematopoietic Stem Cell Transplantation When Compared to Younger PatientsNicholas Prabhakar, Sheila Haugh, Leonard Klein, Tulio Rodriguez, Jacob Bitran*. Elderly (> 70 years) Multiple Myeloma Patients Benefit Equally from Autologous Hematopoietic Stem Cell Transplantation When Compared to Younger Patients. J Stem Cell Ther Transplant. 2024: doi: 10.29328/journal.jsctt.1001042; 8: 042.047
-
Using Model Classification to detect Bias in Hospital TriagingPatrick Ting*, Aayaan Sahu, Nishad Wajge, Vineet Rao, Hiresh Poosarla, Phil Mui. Using Model Classification to detect Bias in Hospital Triaging. Ann Biomed Sci Eng. 2023: doi: 10.29328/journal.abse.1001022; 7: 024-030
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024: doi: 10.29328/journal.acr.1001099; 8: 075-077
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."