More Information
Submitted: October 25, 2024 | Approved: April 15, 2025 | Published: April 16, 2025
How to cite this article: Shristi S, Mohanty S, Nayak S, Biswas P, Sahu AK, Naik PK, et al. Prevalence of Anemia among Chronic Kidney Disease and Chronic Kidney Disease of unknown Etiology Patients of Bargarh District Odisha: A Cross Sectional Study. J Clini Nephrol. 2025; 9(3): 049-055.
Available from:
https://dx.doi.org/10.29328/journal.jcn.1001155
DOI: 10.29328/journal.jcn.1001155
Copyright license: © 2025 Shristi S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: CKD; CKDu; Anemia prevalence; Odisha; Bargarh; Pesticides
Prevalence of Anemia among Chronic Kidney Disease and Chronic Kidney Disease of unknown Etiology Patients of Bargarh District Odisha: A Cross Sectional Study
Sourav Shristi1, Sharat Mohanty2,3, Sunanda Nayak4, Pralaya Biswas5,6, Ashish Kumar Sahu5, Pradeep Kumar Naik3 and Iswar Baitharu5*
1Department of Nephrology, Veer Surendra Sai Institute of Medical Sciences and Research, Burla-768019, Odisha, India
2State Pollution Control Board, Bhubaneswar, Odisha, India
3Department of Biotechnology and Bioinformatics, Sambalpur University, Odisha, India
4Department of Pathology, Veer Surendra Sai Institute of Medical Sciences and Research, Burla-768019, Odisha, India
5Renal Toxicopathology & Medicine Laboratory, Department of Environmental Sciences, Sambalpur University, Burla-768019, Odisha, India
6School of Life Sciences, Sambalpur University, Burla-768019, Odisha, India
*Address for Correspondence: Dr. Iswar Baitharu, Assistant Professor-II, Renal Toxicopathology & Medicine Laboratory, Department of Environmental Sciences, Sambalpur University, Burla-768019, Odisha, India, Email: iswarbaitharu@suniv.ac.in
Introduction: Anemia is a known complication in Chronic Kidney Disease (CKD). Previous study carried out by our lab reported higher prevalence of CKD and Chronic Kidney Disease of Unknown Etiology (CKDu) in Bargarh district, Odisha, India and 16 hotspot villages were identified in the district. The present study focused on assessing the prevalence of anemia among CKD and CKDu patients.
Methods: A cross-sectional study was conducted in the CKD hotspot villages of Bargarh district. Random cluster sampling method was used to assess the prevalence of anemia among CKD and CKDu patients. Patients’ history was collected using questionnaire. Blood samples were collected from the participants, and were analyzed for serological and biochemical parameters: such as creatinine, albumin, cholesterol, Complete Blood Count.
Results: In this study higher number of patients with CKDu (89.17%) were found to be anemic than CKD (85.71%) patients. Males were seen to be more prone to anemia and CKDu. Microcytic anemia was noted to be higher in CKDu populace (57.96%). Majority of patients in this study were farmers. Late CKD stages were found to be associated with higher prevalence of anemia. Significant relation of diastolic blood pressure, serum albumin, cholesterol, serum creatinine and serum urea with hemoglobin was observed.
Conclusion: The present study demonstrates a high prevalence of anemia among patients with both CKD and CKDu in the Bargarh district of Odisha, with a notably higher burden observed in the CKDu group. The predominance of microcytic anemia, particularly among male agricultural workers, underscores the possible influence of environmental and occupational factors.
Over the years, Chronic Kidney Disease (CKD) has emerged as a major public health problem and a significant contributor to the overall non-communicable disease burden globally, which accounts for nearly 700 million active cases and 1.2 million deaths per year (GBD Chronic Kidney Disease Collaboration 2020) [1]. Worldwide, the prevalence of CKD varies from 8% to 16% representing over 750 million cases, of which 78% (387.5 million) are from low-middle-income countries [2,3]. In the United States alone, it is estimated that more than 8 million individuals have stage 3 chronic kidney disease, and many more have less severe chronic kidney disease or are at increased risk for development of chronic kidney disease. As per a systematic analysis of global burden of diseases, CKD contributes significantly to global morbidity and also as a risk factor to cardiovascular diseases [4]. However, for more than two decades, various regions of world have experienced an excess of CKD unrelated to these traditional causes, referred to as “CKDu”, particularly in Central America and Mexico (Mesoamerican nephropathy) [5], the North-Central Province of Sri Lanka [6] and in the state of Andhra Pradesh of India (Uddanam endemic nephropathy) [7], Supebeda Chhattisgarh [8]. In recent years there have been increasing reports of CKD and CKDu cases from the State of Odisha, India. Only limited research exploring the contributing factors from this region is available. Day by day the cases of CKD and CKDu are increasing with an increase of disease burden and morbidity in these regions. A preliminary work by our lab has identified 16 villages of Bargarh district, Odisha as hot spot region for CKD. Moreover, the prevalence of CKDu was found to be higher in these hot spot villages in comparison to CKD cases.
Anemia is a common and early complication in patients with Chronic Kidney Disease (CKD) and chronic kidney disease of unknown etiology (CKDu), and it is often associated with poor clinical outcomes. Specific biomarkers such as serum albumin, total cholesterol, and hemoglobin levels have been linked to the presence and severity of anemia in CKD/CKDu patients. Hypoalbuminemia reflects malnutrition and inflammation, both of which contribute to anemia, while low cholesterol levels have been observed in advanced CKD stages and are also associated with poor erythropoietic response [9,10]. The prevalence and severity of anemia increase progressively with CKD stage, with notable declines in hemoglobin concentrations observed from Stage 3 onwards, becoming more pronounced in Stages 4 and 5 due to reduced erythropoietin production and iron dysregulation [11]. CKDu, which lacks traditional risk factors like diabetes or hypertension, also presents with early-onset anemia, underscoring the need for biomarker-based risk evaluation in endemic regions such as Bargarh district in Odisha.
Reduced erythrocyte mass, or anemia, is one of the regular consequences of Chronic Kidney Disease (CKD) because of central role played by erythropoietin (EPO) in the regulation of erythropoiesis [9]. Anemia can manifest itself early in the course of CKD, and its severity and prevalence go in parallel with the progression of kidney disease. It is suggested that CKD had a significant association with anemia and is considered as a possible cause when the glomerular filtration rate (GFR) is < 60mL/min/1.37 m2 and, it is more likely to be the cause if the GFR is < 30 mL/min/1.73 m2 [10]. Given the significance of severe anemia on quality of life among the patients with kidney failure, anemia is considered one of the most clinically significant complications of this disease. Anemia is one of the most common complications of CKD and contributes significantly of cardiovascular diseases and decreases the quality of patients life [11]. The use of iron therapies and erythropoiesis-stimulating agents (ESAs) has shown improvement in patients with anemia of CKD. In India a cross sectional study reported 82.4% of anemia prevalence in CKD patients. However, no study has focused on prevalence of anemia among CKD and CKDu patients in agricultural zone of India and Odisha yet.
After the commissioning of Hirakud Dam the Bargarh district and area nearby to it become highly irrigated resulting in tremendous rise in the Rice cultivation. This increase paralleled the intense use of pesticides, agrochemicals and heavy metals [12,13]. As a consequence, now a days a varied number of health issues are being observed among the populace of the district. Bargarh was regarded as the cancer capital of India but, now the cases of CKD, especially CKDu has reached alarming levels in this region. The present work aims to study the prevalence of anemia among CKD and CKDu patients and its related clinical alteration in patients of Bargarh district which is a noble piece of work that has never been addressed earlier.
Study site
Bargarh district once regarded as the Rice Bowl of the state, situated at the western part of Odisha, India. Geographically located at 21.33 ͦN, 83.62 ͦ E, having a total population of 14,81,255 out of which male constitute 7,49,161 and female 7,32,094, according to census report 2011. The whole district is divided into 12 blocks out of which 8 villages from Bijepur, 2 from Gaisilate, 2 from Bhatli, 1 from Bheden, and 3 villages from Attabira blocks were identified as Hot-Spot for CKD/CKDu cases with prevalence of CKD incidence twice as compare to National average of CKD burden. A vast chunk of populace of district practice agricultural activities. Paddy is the major crop grown in this region. Around 60% of the cultivated land of the district is irrigated under Hirakud Dam Command.
Study population
This cross-sectional study was conducted in those 16 villages of Bargarh District coming under 4 blocks which were identified as Hot-Spots for CKD incidence as stated above, using clustered random sampling and probability proportionate to Size (PPS) methodology. A total of 710 individuals were screened out of which 484 were males and 226 were female participants. The present study includes participants who met inclusion criteria. The inclusion criteria include participants aged 18 years or more and those who agreed to participate in the study and provided written informed consent. Exclusion criteria includes participants who do not agree to take part in study, patients diagnosed with diseases other than CKD that might influence hematological profile (hematological disorders, acute or chronic inflammatory condition, hemorrhagic episode and cancer), and pregnant or lactating women.
Diagnosis criteria and definitions
The diagnosis criteria followed for identifying patients with CKD was based on Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (estimated GFR < 60 ml/min/1.73 m2 for ≥ 3 months and albuminuria).
The diagnosis of Hypertension was based on history (already on treatment) or through screening. The latter being diagnosed if systolic blood pressure is more than or equal to 140 mmHg and/or diastolic blood pressure more than or equal to 90 mmHg on three separate occasions. Diagnosis of diabetes was also based on either previous history (on medications) or through screening random plasma glucose (normal: 70 -140 mg/dl, diabetic: ≥ 150 mg/dl). Anemia was defined if hemoglobin level < 12 g/dl for women and < 13.5 g/ dl for men. Anemia was further categorized into normocytic, microcytic, and macrocytic anemia based on the mean corpuscular volume (MCV). Normocytic anemia was defined as MCV between 80 and 100 fl, microcytic anemia as MCV less than 80 fl, and macrocytic anemia as more than 100 fl. The patients having the history of prolonged hypertension, diabetes and other known etiologies were categorized as CKD group. All other CKD cases with no history of diabetes or hypertension or have mild hypertension and with progressive end stage renal failure, restricted to a specific geographical area, predominance among male and younger population and people with lower income mostly working in hot climatic environment subjected to dehydration were included under Chronic Kidney Disease of Unknown Etiology (CKDu).
Clinical measurements
Arm blood pressure was measured after 5 minutes of rest in the sitting position using an automated clinically validated digital blood pressure monitoring machine (Dr. Morpen, Model- BP 15, India) and an average of three readings was recorded. Height and weight were measured using a standiometer (SECA model 213, Hamburg, Germany) and digital calibrated scales (OMRON Model HN 865), for calculation of Body Mass Index (BMI).
Sample collection and analysis
Before collecting the blood sample, the puncture site was sanitized and cleaned. Blood samples were collected from peripheral veins using three different vacutainer tubes viz., plain tube, EDTA containing tube and Fluoride tube, for the estimation of serological parameters, hemoglobin, complete blood count, and random blood sugar in respective tubes from all participants. Collected blood samples were transported in ice-carrier box to Regional Diagnostic Center, Veer Surendra Sai Institute of Medical Science and Research, Burla, Odisha, India for analysis. The samples were processed and tested on the same day of sample collection. Serum sample were obtained by centrifugation of blood samples at 3000 rpm for 10 minutes at 4oC. Random blood sugar and serological parameters were estimated, using Transasia XL 1000 Auto Analyzer. Photometric method was used to estimate Hemoglobin (Hb) and Complete Blood Count (CBC) using Mindray BC 1000 Six Part. All chemical reagents of analytical grade were purchased from commercially available recognized sources. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was used to calculate the eGFR (mL/min/1.73 m2).
Statistical analysis
Microsoft Excel 2016 was used for data processing and IBM SPSS (version 25.0) was applied for statistical analysis. Data was presented as mean ± standard deviation. Spearman’s rank correlation test was applied to determine the associations between hemoglobin and other parameters. Independent t - Test was applied to test differences in clinical parameters. Moreover, severity-specific scatter plot graphs were used to demonstrate associations between altered blood parameters of CKD and CKDu patients. Significant differences or associations among the parameters were considered at a p - value of less than 0.05.
Demographic characteristics of studied population
A total of 1136 individuals were screened in the hotspot villages using cluster random sampling. Based on inclusion criteria 710 individuals from screened population were included in study, while rest 426 were excluded. Out of 710 individuals, 232 were found to be affected with Chronic Kidney Disease. Among the affected individuals, 77 were diagnosed with CKD while rest 157 were found to be affected with CKDu. Out of 77 CKD patients, 66 patients were found to be suffering from anemia whereas in case of CKDu, 140 out of 157 CKDu patients were found to be anemic. In the present study, number of male CKD and CKDu patients affected with anemia was observed to be very high compared to females indicating higher vulnerability of males toward CKD and anemia. No significant variation in age and BMI was observed between anemic CKD and CKDu patients in the studied population. Occupational analysis of the studied population indicated that the majority of the affected anemic male CKD and CKDu patients were actively practicing farming of rice and other crops for long time and had long history of pesticide application in their crop field. The prevalence of CKD and CKDu with anemia was found to be higher among illiterate and lower income groups. A detailed demographic character of studied participants has been presented in Table 1.
| Table 1: Demographic characteristic of CKD patients included in study (N = 232) | ||||
| Demographic Characteristic | Anemic CKD Patients (N = 66) | Anemic CKDu Patients (N = 140) | Non Anemic CKD patients (N = 9) | Non Anemic CKDu patients (N = 17) |
| Age (Mean ± SD) | 48 ± 10.8 | 54 ± 12.38 | 49 ± 16.2 | 50 ± 11.47 |
| No. of Male | 42 | 84 | 3 | 11 |
| No. of Female | 24 | 56 | 6 | 6 |
| Hypertension | 23 | Nil | 4 | Nil |
| Diabetes | 43 | Nil | 17 | Nil |
| Body Mass Index (Mean ± SD) | 26 ± 5.8 | 25 ± 3.11 | 21 ± 5.22 | 292.14 |
| Occupation | ||||
| Farmers | 32 | 98 | 2 | 5 |
| Labors | 20 | 26 | 7 | 8 |
| Others | 14 | 16 | 0 | 4 |
| Level of Education | ||||
| Illiterates | 27 | 56 | 5 | 11 |
| Primary | 24 | 36 | 3 | 4 |
| Degree | 15 | 32 | 1 | 2 |
| Upper Primary | 0 | 16 | 0 | 0 |
| Income | ||||
| High | 06 | 12 | 0 | 0 |
| Average | 28 | 56 | 0 | 5 |
| Low | 32 | 72 | 9 | 12 |
| History of Tobacco use | 28 | 11 | 0 | 0 |
| History of Alcohol use | 34 | 6 | 2 | 3 |
Clinical parameters
Significant difference in age, random plasma glucose, serum sodium, and serum uric acid was observed between anemic CKD and anemic CKDu patient as shown in Table 2. No major difference in parameters such serum creatinine, serum urea, LDL, HDL, Triglyceride was noted in CKD and CKDu patients suffering from anemia.
| Table 2: Comparisons of clinical data of CKD and CKDu patients with anemia. | |||
| Parameters | Anemic CKD Patients | Anemic CKDu Patients | p value |
| Age | 58.04 ± 10.84 | 54.9 ± 12.38 | 0.018 |
| Systolic BP | 145.98 ± 23.79 | 117.68 ± 17.37 | 0.065 |
| Diastolic BP | 80.40 ± 14.42 | 68.92 ± 15.83 | 0.844 |
| RBC | 3.56 ± 0.68 | 3.61 ± 0.72 | 0.977 |
| HGB | 9.14 ± 1.51 | 9.11 ± 1.60 | 0.328 |
| HCT | 27.48 ± 4.35 | 27.42 ± 5.05 | 0.226 |
| MCV | 75.97 ± 15.92 | 81.78 ± 68.88 | 0.615 |
| Random Plasma Glucose | 140.42 ± 63.96 | 101.02 ± 16.25 | 0.000 |
| Serum Urea | 48.57 ± 14.26 | 50.23 ± 18.39 | 0.151 |
| Serum Creatinine | 2.85 ± 1.37 | 3.07 ± 2.14 | 0.252 |
| Serum Albumin | 3.74 ± 0.35 | 4.11 ± 3.47 | 0.305 |
| Serum Sodium | 137.90 ± 4.94 | 139.58 ± 4.98 | 0.009 |
| Serum Potassium | 4.25 ± 0.98 | 4.23 ± 0.85 | 0.455 |
| Serum Uric Acid | 8.46 ± 1.89 | 13.76 ± 29.56 | 0.031 |
| Serum Cholesterol | 145.3 ± 41.33 | 139.64 ± 38.34 | 0.855 |
| HDL | 41.86 ± 12.49 | 41.62 ± 12.84 | 0.711 |
| LDL | 74.78 ± 27.97 | 70.30 ± 24.63 | 0.094 |
| Triglyceride | 135.64 ± 72.67 | 138.18 ± 78.46 | 0.591 |
| eGFR | 20.61 ± 7.91 | 20.57 ± 8.48 | 0.960 |
CKD stage and anemia
The prevalence of anemia was found to be increased with progress in the stages of CKD. 60.71% of CKDu anemic patients were at stage IV which is highest as compared to other stages followed by patients at stage V and III, a similar trend was also seen in anemic CKD group reporting 68.1% of patients in stage IV followed by 21.2% and 10.6% of patients in stage V and III respectively, while no patients were found at any of the group at stage I and II. Anemic CKDu group has more number of patients i.e. 85 at stage IV in comparison to anemic CKD patients at the same stage (Table 3).
| Table 3: Distribution of study population according to CKD stage. | ||||
| Stages of CKD | Anemic CKD Patients N(%) |
Anemic CKDu Patients N(%) |
Non Anemic CKD patients N(%) |
Non Anemic CKDu patients N(%) |
| Stage I | 0(0) | 0 (0) | 0(0) | 0 (0) |
| Stage II | 0(0) | 0(0) | 0(0) | 0 (0) |
| Stage III | 7 (10.6) | 19 (13.57) | 2 (22.2) | 8 (47.05) |
| Stage IV | 45 (68.1) | 85 (60.71) | 6 (66.6) | 7 (41.17) |
| Stage V | 14 (21.2) | 36 (25.71) | 1 (11.1) | 2 (11.76) |
Type of anemia
A major chunk of individuals having CKDu was found to be affected with anemia i.e. 89.17% as compared to that of CKD individuals. Microcytic anemia was found to be highest in CKDu group than the other two types (Normocytic and Macrocytic) in comparison to CKD group. None of the individuals with CKD was found to have Macrocytic anemia while 40% of CKD group and 47% of CKDu group had Normocytic anemia as illustrated in Table 4.
| Table 4: Comparison of hematological abnormalities in respect to anemia between CKD and CKDu group. | ||
| Variables | CKD N = 75 n (%) | CKDu N = 157 n (%) |
| Anemia | 66 (88) | 140 (89.17) |
| Microcytic | 36 (48) | 91 (57.96) |
| Normocytic | 30 (40) | 47 (29.93) |
| Macrocytic | 0 (0) | 2 (1.27) |
| Non-Anemic | 9 (12) | 17 (10.82) |
Correlation of Hemoglobin and other clinical parameters
Significant positive correlation between Diastolic Blood Pressure, serum albumin, eGFR, serum cholesterol with hemoglobin was observed among the anemic CKDu patients while in case of anemic CKD patients, significant positive correlation was noted only for serum albumin and eGFR. Serum urea, serum creatinine, serum potassium and serum uric acid were negatively correlated with hemoglobin level in case of both the studied groups however significant negative correlation of serum creatinine and urea was observed with hemoglobin level in CKDu anemic patients. In both the groups, stage IV had positive correlation while stage III had negative correlation with hemoglobin level, but stage V showed negative correlation in CKD group while positive correlation in CKDu group with hemoglobin level. None of the correlation between hemoglobin concentration and stages of CKD were statistically significant as shown in Table 5.
| Table 5: Correlation between hemoglobin and other parameters for anemic CKD and anemic CKDu patients. | ||||
| Variables | Correlation with hemoglobin in anemic CKD patients (N = 66) | p value | Correlation with hemoglobin in anemic CKDu patients (N = 140) | p value |
| Age | - 0.020 | 0.87 | -0.008 | 0.92 |
| Systolic BP | 0.053 | 0.67 | 0.153 | 0.07 |
| Diastolic BP | 0.182 | 0.14 | 0.189* | 0.02 |
| Random Plasma Glucose | 0.078 | 0.53 | -0.108 | 0.20 |
| Serum Urea | -0.074 | 0.55 | -0.314** | 0.00 |
| Serum Creatinine | -0.11 | 0.36 | -0.316** | 0.00 |
| Serum Albumin | 0.47** | 0.00 | 0.352** | 0.00 |
| eGFR | 0.25* | 0.03 | 0.376** | 0.00 |
| Serum Sodium | 0.041 | 0.74 | 0.01 | 0.90 |
| Serum Potassium | -0.039 | 0.75 | -0.048 | 0.57 |
| Serum Uric Acid | -0.102 | 0.41 | -0.029 | 0.73 |
| Serum Cholesterol | 0.112 | 0.37 | 0.187* | 0.02 |
| Serum HDL | 0.037 | 0.76 | 0.058 | 0.49 |
| Serum LDL | 0.139 | 0.26 | 0.138 | 0.10 |
| Serum Triglyceride | 0.186 | 0.13 | 0.10 | 0.21 |
| Stages Of CKD | ||||
| Stage I | 0 | 0 | 0 | 0 |
| Stage II | 0 | 0 | 0 | 0 |
| Stage III | -0.214 | 0.64 | -0.243 | 0.31 |
| Stage IV | 0.254 | 0.09 | 0.066 | 0.54 |
| Stage V | -0.463 | 0.09 | 0.203 | 0.23 |
| **. Correlation is significant at the 0.01 level (2-tailed). *. Correlation is significant at the 0.05 level (2-tailed). |
||||
Epidemiological studies conducted worldwide recorded higher prevalence of anemia among CKD patients viz. 53.4% in USA, 53.5% in Ethiopia, 44.9% in Korea, 33% in Tanzania, and 6.76% in UK, similar studies in Indian settings by Zaawari, et al. also reported 82.4% prevalence of anemia among the CKD patients [9]. To date no other studies have focused on the epidemiology of anemia among CKD and CKDu patients in India, especially in the state of Odisha. The present study reports relatively higher prevalence of anemia in CKDu cases i.e. 89.17% in compare to that of anemic CKD cases (85.71%) at the hotspot villages of Bargarh district, Odisha. In CKD the anemia is reported to cause due to decrease in iron level and also because of the inability of kidney to produce erythropoietin which is essential of the erythropoiesis [14]. The trend of anemia was found to differ gender wise, in the current study 60% of males suffering from CKDu were found to be anemic while only 40% females having CKDu were anemic, this gender difference may be due to occupational exposure of males to pesticides in agricultural lands. As pesticides are known to have potential nephrotoxic effect that causes CKDu [15]. The majority of the studied population practicing farming were found to be affected with CKDu and also the prevalence of anemia is higher among them which suggests a possible link to the linkage of anemia and pesticide exposure among CKDu patients. Individuals with lower income and limited education were found to be more affected by these disease conditions, previous studies also show higher incidence of CKD and anemia among people with poor socio-economic status. Globally, studies have indicated that populations in developing countries has higher incidence of CKD [16].
In our study the prevalence of anemia has increased with the progression of CKD stages. A similar effect was observed in large-scale, cross-sectional, US multicenter survey, where the prevalence was found to be increased with the decline in renal function. Evidence from Chinese cross-sectional study also reported the similar observation [17], the prevalence of anemia increased from 22.4% to 79.2% from stage-I CKD to Stage-IV CKD. This increasing incidence of anemia among CKD patients may be attributed to reduced RBC life span as erythropoietin production get decline with progression of the staging. In this present study, percentage of anemic patients has increased from stage III to stage IV accounting 13.57% and 60.71% of anemic individuals in CKDu at stage III and IV respectively, while 10.6% and 21.2% of anemic individuals in CKD group at stage III and IV respectively. But in the late stage i.e. at stage V number of anemic individuals has reduced considerably both in cases of CKD and CKDu. These decrease in the number of patients in late stage may be due to mortality of patients in the study area, because of poor health facility, late diagnosis, illiteracy or poverty. The study result shows that anemic CKDu patients at stage IV are comparatively more than that of anemic CKD patients at the same stage, however these difference in number of patients in both groups may be due to variability in patients number.
In the present study we observed higher cases of iron deficiency anemia i.e. microcytic anemia in CKDu group than CKD. Previous studies shows that CKD patients have an absolute iron deficiency. This has several causes such as decrease absorption of iron in body due to lower hepcidin level in CKD patients. Hepcidin is a small hormone peptide secreted by liver which help in absorption of iron and its level decrease with decrease in GFR, the higher rate of iron loss in CKD is also attributed to increased blood loss during dialysis, frequent phlebotomies, and gastrointestinal bleeding. Moreover, some of the organochlorin pesticides were also reported to cause anemia. Since the majority of agricultural practitioners were affected with CKDu and also the cases of microcytic anemia are prevalent among them it can be deduced that pesticides exposure may be linked towards the development of microcytic anemia among CKDu patients in the present study.
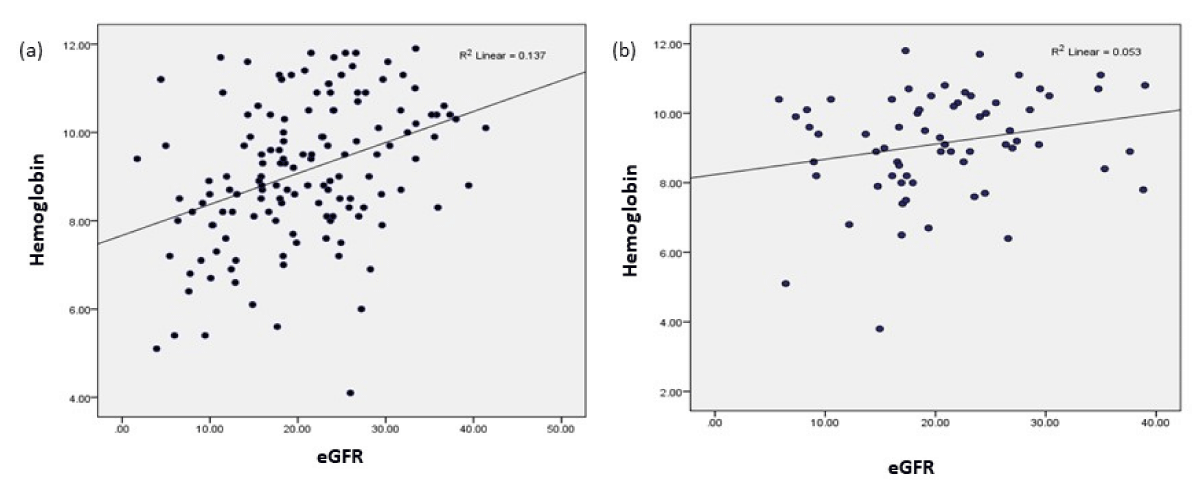
This study reports negative correlation of serum urea and creatinine with hemoglobin level in cases of both CKD and CKDu group which is in line with past studies. We also observed a significant positive liner correlation of diastolic BP with the level of hemoglobin in anemic CKDu population. It is known that in sever anemic condition, body produce some hormones that causes vaso-constriction which causes rise in diastolic blood pressure. In present study, eGFR was directly correlated with the hemoglobin and inversely correlated with the serum creatinine level. A similar finding was reported by Kazmi, et al. in a retrospective cohort study [18].This finding was also supported by Feteh, et al. and New, et al. they also found a linear relation between eGFR and hemoglobin level. In this study a strong linear correlation was observed between eGFR and hemoglobin level in anemic CKDu population while comparatively less significant positive correlation was depicted between the two among anemic CKD population as illustrated in Figure 1 and detailed in Table 5.
Figure 1: Correlation between eGFR and Hemoglobin (a) Anemic CKDu, (b) Anemic CKD.
Dyslipidemia is well-documented in CKD patients, past studies revealed increased cholesterol level in patients with CKD and anemia [19]. Our study also reports a liner positive relationship of cholesterol with hemoglobin level in both CKD and CKDu population, however the result was significant in case of CKDu anemic patients. CKD is associated with dyslipidemia comprised of elevated triglycerides and low HDL-cholesterol and thus, total cholesterol are generally not elevated however proteinuria correlates with cholesterol and triglycerides. CKD leads to downregulation of lipoprotein lipase and LDL-receptor, and increased triglycerides in CKD are due to delayed catabolism of triglyceride rich lipoproteins, with no difference in production rate and with the worsening of CKD the dyslipidemia condition increases which results in an increased risk of Cardiovascular Diseases (CVD) in patients with CKD and anemia in progression with CKD stages [20].
In light of the high prevalence of anemia among CKD and CKDu patients in the Bargarh district, it is imperative to implement targeted public health interventions. Given the region’s agrarian nature and potential environmental exposures, particularly to agrochemicals, there is a pressing need to promote safer agricultural practices, including the regulated use of pesticides, provision of protective equipment, and training on chemical handling and storage [21-24]. Additionally, community-based awareness programs should be launched to educate farmers and rural populations about the early signs of CKD/CKDu, the importance of regular health screenings, and the role of nutritional biomarkers like serum albumin and cholesterol in anemia management. Policy-level actions should also focus on developing surveillance systems, ensuring access to clean drinking water, and incorporating CKD screening into routine primary care in endemic regions [25] Integrating these recommendations into existing rural health and agricultural extension frameworks could significantly reduce disease burden and improve early diagnosis and anemia management among at-risk populations.
While this study provides valuable insights into the prevalence of anemia among CKD and CKDu patients in the Bargarh district, several limitations should be acknowledged. This study should be interpreted in light of certain limitations. First this cross-sectional study design does not allow drawing true associations between anemia and their causal factors. Second, results cannot be generalized to the entire Indian population as the patients were selected from a single center. Third, the reticulocyte count, level of iron, erythropoietin level and hemoglobinopathies were not assessed in the studied population. Last, we cannot rule out the possibility of confounding effects of comorbidity, concomitant medication, and lifestyle factors on the diagnosis of anemia in our study. Nevertheless, this is first of its kind study, which has focused on comparative epidemiology of anemia in person with CKD and CKDu in Indian population. Future studies should aim for larger community-based cohorts, include longitudinal designs, and incorporate environmental and occupational exposure analysis for a more comprehensive understanding.
The present study demonstrates a high prevalence of anemia among patients with both CKD and CKDu in the Bargarh district of Odisha, with a notably higher burden observed in the CKDu group. The predominance of microcytic anemia, particularly among male agricultural laborers, underscores the possible influence of environmental and occupational factors—such as prolonged exposure to agrochemicals—in the etiology of CKDu and its hematological complications. The significant associations between hemoglobin levels and biochemical parameters such as serum albumin, creatinine, cholesterol, urea, and blood pressure further highlight the multifactorial nature of anemia in these patients. Given the high representation of Stage IV and V CKD among anemic individuals, early detection and monitoring of anemia should be part of standard CKD/CKDu management in endemic areas. Public health strategies focusing on community awareness, safe farming practices, nutritional support, and regular health screening are critical to mitigate the progression of CKDu and its complications. Future longitudinal and mechanistic studies are warranted to explore the causal pathways and to validate the role of environmental exposures in CKDu-associated anemia.
Ethical approval
The study was approved by the Institutional Ethical Committee of Sambalpur University (IEC-SU), Odisha vide letter number 16/IEC-SU/2023, dated 29/07/2023.
The authors acknowledge Veer Surendra Sai Institute of Medical Science and Research, Burla to facilitate the sample testing. We also acknowledge CDMO, and all staffs of district head quarter hospital, Bargarh and the district administration of Bargarh, Odisha.
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7–11. Available from: https://doi.org/10.1016/j.kisu.2021.11.003
- George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB, Kengne AP. Chronic kidney disease in low-income to middle-income countries: The case for increased screening. BMJ Glob Health. 2017;2(2):e000256. Available from: https://doi.org/10.1136/bmjgh-2016-000256
- Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2):e000380. Available from: https://doi.org/10.1136/bmjgh-2017-000380
- Bikbov B, Purcell C, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. Available from: https://doi.org/10.1016/s0140-6736(20)30045-3
- Wesseling C, Crowe J, Hogstedt C, Jakobsson K, Lucas R, Wegman DH. Mesoamerican Nephropathy: Report from the First International Research Workshop on MeN. Am J Kidney Dis. 2013;63(3):396–404. Available from: https://doi.org/10.1053/j.ajkd.2013.08.014
- Chandrajith R, Nanayakkara S, Itai K, Aturaliya TNC, Dissanayake CB, Abeysekera T, et al. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ Geochem Health. 2011;33(3):267–78. Available from: https://doi.org/10.1007/s10653-010-9339-1
- Tatapudi RR, Rentala S, Gullipalli P, Komarraju AL, Singh AK, Tatapudi VS, et al. High Prevalence of CKD of Unknown Etiology in Uddanam, India. Kidney Int Rep. 2019;4(3):380–9. Available from: https://doi.org/10.1016/j.ekir.2018.10.006
- Chowdhary P, Rathore V, Jain K, Galhotra A, Verma N, Kale SA, et al. CKD of Unknown Origin in Supebeda, Chhattisgarh, India. Kidney Int Rep. 2021;6(1):210–4. Available from: https://doi.org/10.1016/j.ekir.2020.10.007
- Pisoni RL, Bragg-Gresham JL, Young EW, Akizawa T, Asano Y, Locatelli F, et al. Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2004;44(1):94–111. Available from: https://doi.org/10.1053/j.ajkd.2004.03.023
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med. 2002;162(12):1401–8. Available from: https://doi.org/10.1001/archinte.162.12.1401
- Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1):e84943. Available from: https://doi.org/10.1371/journal.pone.0084943
- Zaawari A, Tejaswini KL, Davina GD, Singanaveni A. Prevalence of anemia among chronic kidney disease patients in India: a single-centre study. Int J Basic Clin Pharmacol. 2022;11(5):404–9. Available from: https://doi.org/10.18203/2319-2003.ijbcp20222135
- Levin A, Stevens PE, Bilous RW, Coresh J, De Francisco ALM, De Jong PE, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. Available from: https://pure.johnshopkins.edu/en/publications/kidney-disease-improving-global-outcomes-kdigo-ckd-work-group-kdi-4
- Sathyan S, George S, Vijayan P. Prevalence of anemia and cardiovascular diseases in chronic kidney disease patients: a single tertiary care centre study. Int J Adv Med. 2017;4(1):247–51. Available from: https://doi.org/10.18203/2349-3933.ijam20170120
- Mishra SS, Sahu KA, Dungdung SM, Ahmed NS, Baitharu I. Pesticide pollution in freshwater and its impact on community health. In: Current Developments in Biotechnology and Bioengineering: Pesticides: Human Health, Environmental Impacts and Management. 2023.
- Sahu AK, Dung MSD, Sahoo SK, Mir SA, Nayak B, Baitharu I. Ecological and human health risk associated with heavy metals in sediments and bioaccumulation in some commercially important fishes in Mahanadi River, Odisha, India. Environ Chem Ecotoxicol. 2023;5:100394. Available from: http://dx.doi.org/10.1016/j.enceco.2023.08.001
- Cernaro V, Coppolino G, Visconti L, Rivoli L, Lacquaniti A, Santoro D, et al. Erythropoiesis and chronic kidney disease–related anemia: From physiology to new therapeutic advancements. Med Res Rev. 2019;39(1):427–60. Available from: https://doi.org/10.1002/med.21527
- De Broe ME, Vervaet BA. Is an Environmental Nephrotoxin the Primary Cause of CKDu (Mesoamerican Nephropathy)? PRO. Kidney360. 2020;1(7):591–5. Available from: https://doi.org/10.34067/KID.0003172020
- Bello AK, Peters J, Rigby J, Rahman AA, El Nahas M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3(5):1316–23. Available from: https://doi.org/10.2215/cjn.00680208
- Shen Y, Wang J, Yuan J, Yang L, Yu F, Wang X, et al. Anemia among Chinese patients with chronic kidney disease and its association with quality of life - results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol. 2021;22(1):64. Available from: https://doi.org/10.1186/s12882-021-02247-8
- Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, et al. Anemia: An early complication of chronic renal insufficiency. Am J Kidney Dis. 2001;38(4):803-812. https://doi.org/10.1053/ajkd.2001.27699
- Pavanello C, Ossoli A. HDL and chronic kidney disease. Atheroscler Plus. 2023;52:9–17. Available from: https://doi.org/10.1016/j.athplu.2023.04.001
- Liang X, Ye M, Tao M, Zheng D, Cai R, Zhu Y, et al. The association between dyslipidemia and the incidence of chronic kidney disease in the general Zhejiang population: A retrospective study. BMC Nephrol. 2020;21(1):252. Available from: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-020-01907-5
- Jayasumana C, Paranagama P, Agampodi S, Wijewardane C, Gunatilake S, Siribaddana S. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health. 2015;14(1):6. Available from: https://link.springer.com/article/10.1186/1476-069x-14-6
- Singh AK, Farag YMK, Mittal BV, Subramanian KK, Reddy SRK, Acharya VN, et al. Epidemiology and risk factors of chronic kidney disease in India - Results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol. 2013;14(1):114. Available from: https://link.springer.com/article/10.1186/1471-2369-14-114