More Information
Submitted: March 08, 2025 | Approved: March 18, 2025 | Published: March 19, 2025
How to cite this article: Zerouali S, Chouhani A, Himafi M, Zaryouhi M, El Bardai G, Allata Y, et al. Posterior Reversible Encephalopathy Syndrome and Systemic Lupus Erythematosus: An Underestimated Association Case Report and Review of Pathophysiological Mechanisms. J Clini Nephrol. 2025; 9(3): 041-045. Available from:
https://dx.doi.org/10.29328/journal.jcn.1001153
DOI: 10.29328/journal.jcn.1001153
Copyright license: © 2025 Zerouali S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posterior Reversible Encephalopathy Syndrome and Systemic Lupus Erythematosus: An Underestimated Association Case Report and Review of Pathophysiological Mechanisms
Zerouali S1*, Chouhani A1, Himafi M1, Zaryouhi M2, El Bardai G1, Allata Y1, Kabbali N1, Elbouardi N3, Tahri L2 and Sqalli Houssaini T1
1Nephrology Department, Hassan II University Hospital Fes, Morocco
2Anatamo-pathology Department, Hassan II University Hospital Fes, Morocco
3Radiology Department, Hassan II University Hospital Fes, Morocco
*Address for Correspondence: Zerouali S, Nephrology Department, Hassan II University Hospital Fes, Morocco, Email: dr.sarazerouali@gmail.com
Posterior Reversible Encephalopathy Syndrome (PRES) is a rare clinico-radiological entity, and its global incidence remains unknown. It can be observed in various clinical circumstances, notably during Systemic Lupus Erythematosus (SLE). Its diagnosis is radiological, characterized by the appearance of vasogenic edema within the white matter, predominantly in the posterior parieto-occipital regions. The occurrence of PRES in the context of SLE is rare. Brain MRI with diffusion sequences is the examination of choice for diagnosis and follow-up. Controlling hypertension is the cornerstone of treatment and helps minimize sequelae. The objective of our study is to report a clinical case of a patient followed for lupus nephropathy who developed PRES and to describe pathophysiological mechanisms.
We report a case of a 46-year-old female patient with a history of SLE, admitted with impaired consciousness, convulsive seizures, and a hypertensive peak of 170/110 mmHg. Brain imaging confirmed the diagnosis of PRES, reinforcing the clinical suspicion. Treatment mainly consisted of managing arterial hypertension and preventing recurrent seizures. The patient fully recovered without any persistent neurological deficits. PRES is a rare condition, and appropriate management of its clinical manifestations is crucial to prevent both clinical and radiological sequelae.
Posterior Reversible Encephalopathy Syndrome (PRES) is a clinico-radiological entity first described by Hinchey, et al. in 1996 [1]. Its clinical and radiological signs are usually reversible [1]. Seizures occur in at least 40% of PRES patients.
Clinical presentation includes symptoms such as headaches, visual disturbances, nausea, and vomiting, which can progress to neurological deficits or impaired consciousness [1].
PRES can occur in patients with Systemic Lupus Erythematosus (SLE), though its pathophysiology remains complex. Brain MRI remains the gold standard for diagnosis, although a brain CT scan can also confirm the diagnosis in 50% of cases [1].
The primary goal of treatment is initially preventive and subsequently curative, focusing on treating the underlying cause and maintaining blood pressure stability [1].
This is a 46-year-old female patient with a history of miscarriage, followed for systemic lupus erythematosus (SLE) with joint involvement (inflammatory-like arthralgia), hematological involvement (hemoglobin at 10 g/dL), and renal involvement (24-hour proteinuria of 6 g/24h, impaired renal function with creatinine levels at 60 mg/L and urea at 1.6 g/L). Immunological tests showed consumed C3 complement, positive antinuclear antibodies, and positive anti-DNA antibodies. Renal biopsy confirmed lupus nephropathy, classified as class 4, with an activity index of 7 and a chronicity index of 4 (Figures 1-4).
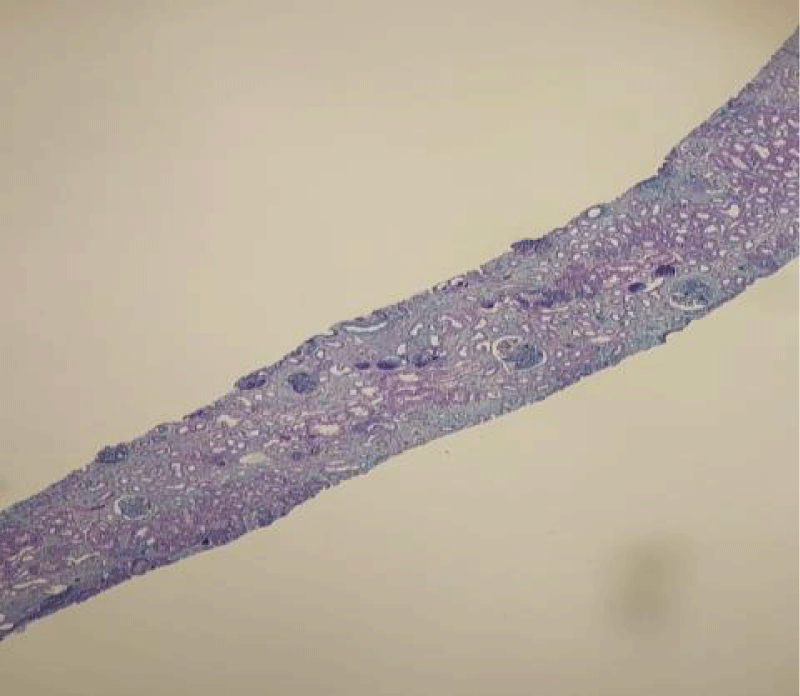
Figure 1: Magnification x4: Masson's trichrome stain 13 glomeruli, including 1 sealed capsule.
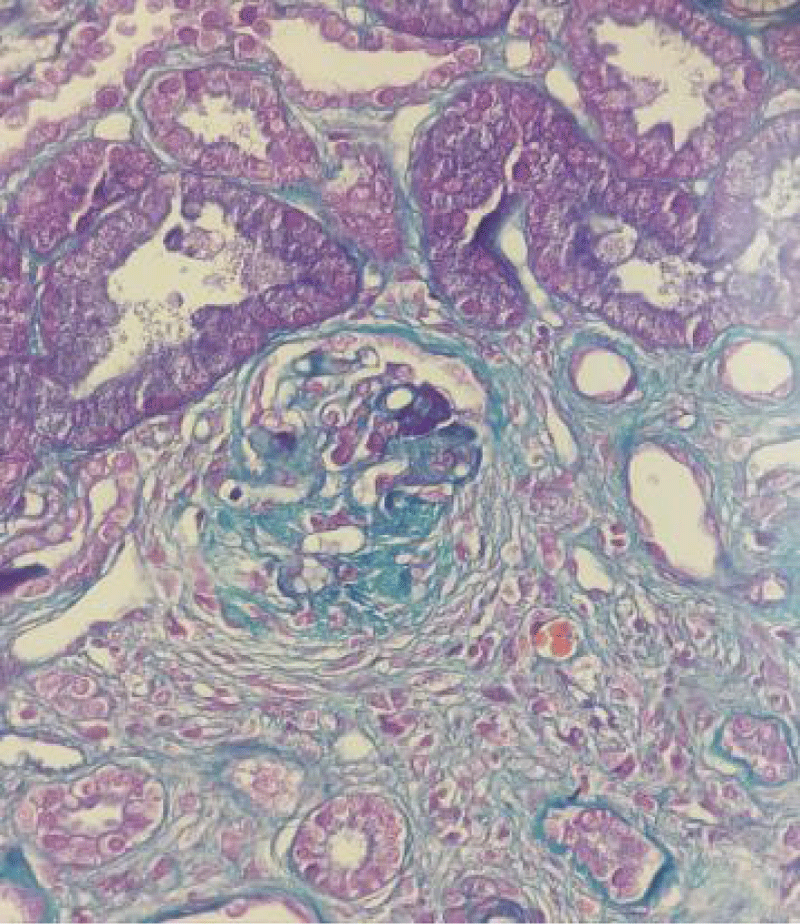
Figure 2: Magnification x20: Masson's trichrome stain: Tubular atrophy estimated at 40% (score 2), interstitial fibro-edema at 10% (score 1), and an inflammatory infiltrate of 20%, consisting of lymphocytes and macrophages (score 1).
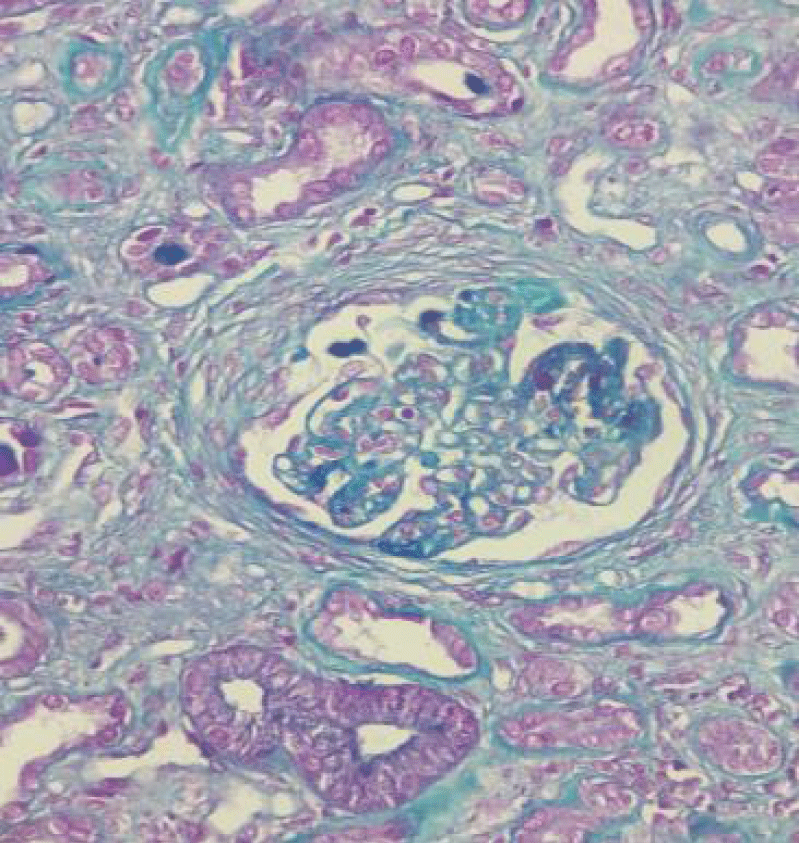
Figure 3: Magnification x20: Masson's trichrome stain: Presence of segmental endocapillary proliferation in 4 glomeruli (score 2).
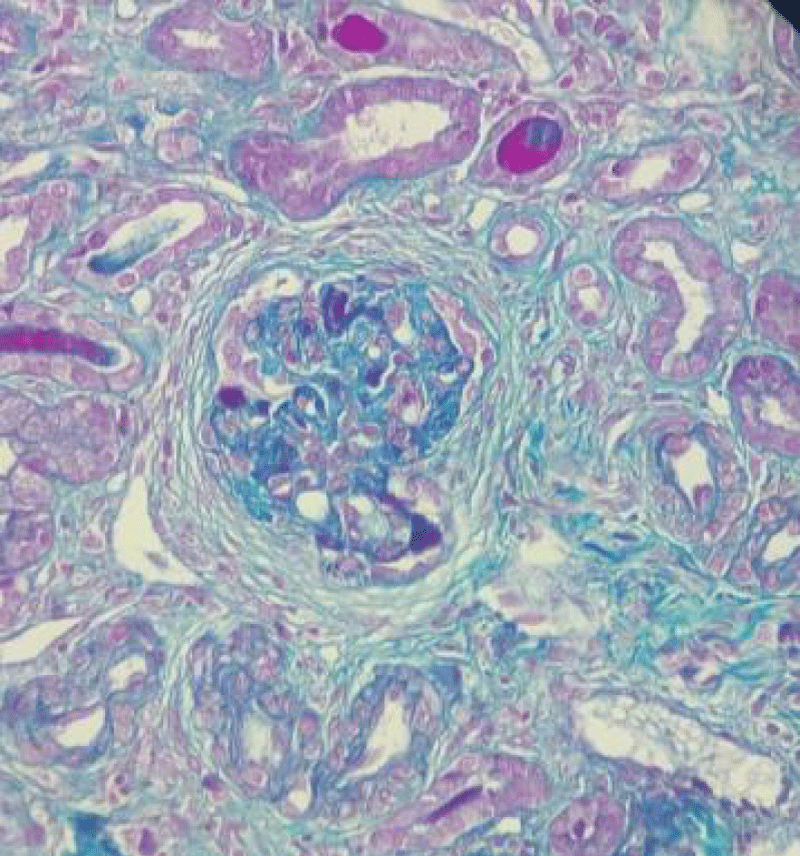
Figure 4: Magnification x20: Masson's trichrome stain: Presence of cellular extracapillary proliferation in one glomerulus and fibro-cellular proliferation in two glomeruli (score 2). Thin basement membrane.
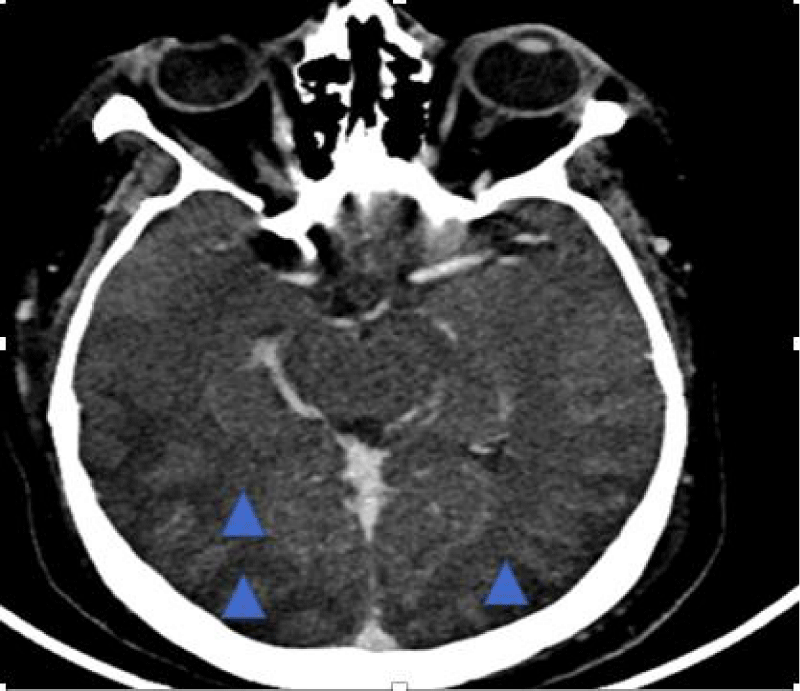
She was treated according to the NIH protocol and received two cycles of cyclophosphamide. The patient required three sessions of dialysis due to symptomatic hyperuremia. She was admitted to the emergency department for a tonic-clonic seizure. Clinically, she presented with impaired consciousness, a blood pressure of 170/110 mmHg, and an oxygen saturation of 92% under 10 liters of O₂. She was treated with two anticonvulsants (phenobarbital and benzodiazepine) and nicardipine. A biological workup was performed, which did not indicate a metabolic origin for the neurological impairment. A brain CT scan was performed, revealing hypodense cortical and subcortical areas in the bilateral and symmetrical posterior fronto-parieto-temporo-occipital regions, with associated cortical sulcus effacement. A hypodense capsulo-lenticular area suggestive of vascular origin was also noted. These findings were consistent with PRES (Figure 5).
Figure 5: Brain CT scan revealing hypodense subcortical areas bilaterally and symmetrically in the posterior fronto-parieto-temporo-occipital regions, with cortical sulcus effacement. Cerebral veins and dural sinus are permeable
Moreover, there were no neurological signs suggestive of neuro-lupus. Four days later, her level of consciousness improved, and she did not experience further seizures. She was discharged on antihypertensive treatment, corticosteroid therapy, sodium valproate, and benzodiazepine. Three months later, the patient was fully conscious, showed no neurological signs, and had stable blood pressure under amlodipine 10 mg and an ACE inhibitor 10 mg.
PRES is a rare condition with an unknown global incidence [2]. It is a clinico-radiological syndrome that can present with neurological and cardinal symptoms such as nausea, vomiting, isolated tonic-clonic seizures, or impaired consciousness [3]. These symptoms are more commonly associated with renal insufficiency, particularly of glomerular origin in the context of systemic diseases (systemic lupus erythematosus, eclampsia, organ transplantation, and bone marrow transplantation), autoimmune diseases, or the use of immunosuppressive treatments (tacrolimus, cyclosporine) [4]. In our case, the patient was admitted with impaired consciousness and a tonic-clonic seizure episode while being followed for Systemic Lupus Erythematosus (SLE).
Pathophysiology
Cerebral blood flow must remain constant despite variations in blood pressure. A decrease in cerebral arterial pressure leads to vasodilation to increase capillary flow. Conversely, an increase in blood pressure results in vasoconstriction, reducing blood flow. Cerebral blood flow ensures normal brain perfusion when blood pressure ranges between 60 mmHg and 140 mmHg [5]. The clinical manifestations and radiological findings of PRES are explained by two main hypotheses:
- First hypothesis [6]: Hypertension leads to an imbalance between vasodilatory and vasoconstrictive mechanisms, resulting in the breakdown of the blood-brain barrier. This disruption allows fluid to leak from blood vessels into the brain parenchyma, contributing to the formation of vasogenic cerebral edema.
- Second hypothesis [7]: Immune system activation causes endothelial cell damage, vasospasm, and cerebral ischemia. These pathophysiological mechanisms are responsible for the development of cytotoxic edema.
However, this hypothesis does not fully explain why hypertension typically precedes the development of PRES [8].
Endothelial cells play an interface role between blood cytokines and leukocytes [9]. Patients with LEAD and high systemic activity are at greater risk of developing PRES [10].
A high SLEDAI score is associated with an increase in pro-inflammatory cytokines such as TNF alpha, IL-1, IL-6, and interferon INF alpha [11,12]. These inflammatory cytokines lead to changes in antigen surfaces (such as P-selectin, E-selectin, ICAM-1, VCAM-1) by reducing leukocyte adhesion, resulting in altered microcirculation and cerebral hypoperfusion [13-16]. Type 1 INF is associated with this endothelial dysfunction [17].
Three elements are involved in the occurrence of PRES in patients with LEAD: endothelial system activation, leukocyte alteration, and microcirculation alteration. These three elements may not be combined to alter cerebral perfusion [13]. This explains why PRES can occur in patients with LEAD without necessarily having high blood pressure readings [14].
Neuro-lupus is associated with an alteration of the blood-brain barrier, leading to a modification in the permeability between the systemic circulation and cerebral circulation. This can be evaluated by measuring the albumin levels in Cerebrospinal Fluid (CSF) as well as by testing IgG levels [18,19]. There are no studies confirming a significant link between the antibodies involved in LEAD and the development of PRES [20]. Brain MRI is considered the gold standard for diagnosis [8]. Cerebral edema can be diagnosed with a non-contrast brain scan, while brain MRI with T2 FLAIR sequence is more sensitive for making the diagnosis [21]. Imaging typically shows a lesion that is iso- or hypo-intense on T1 and hyper-intense on T2 in FLAIR sequence. Enhancement after contrast injection is rare. The lesions are diffuse and predominantly affect the posterior regions: frontal lobe 68%, temporal lobe 40%, and cerebellar hemispheres 30% [21]. Loss of regulation in the cerebral autoregulatory system is responsible for the development of vasogenic edema, which is often reversible. Some studies have utilized cerebral angiography in PRES patients to explore the underlying pathophysiological mechanisms. This radiological examination concluded that there was diffuse and focal vasoconstriction and focal vasodilation, even in patients with borderline blood pressure. There was also a reduction in cerebral blood volume. Vasogenic edema was less significant in hypertensive patients than in normotensive patients due to the chronic adaptation mechanism of intravascular hypertension. This suggests that an initial endothelial lesion precedes vascular damage, ultimately causing cerebral hypoperfusion, edema formation, and potential ischemia. Cerebral angio-MRI helps to rule out cerebral venous thrombosis, which is the main differential diagnosis. The severity of lesions observed on CT and brain MRI is not correlated with the severity of clinical signs [21,22].
In our case, the patient underwent a brain CT scan that showed hypodense cortical-subcortical areas, posterior bilateral and symmetric fronto-parieto-temporo-occipital regions, erasing the cortical sulci in the corresponding areas. There was also the presence of a hypodense capsulo-lenticular area with a vascular appearance.
In this context, we consider a Posterior Reversible Encephalopathy Syndrome (PRES) or a cerebral venous thrombosis. Based on the clinical presentation and the CT scan, the diagnosis of PRES was evident. A brain MRI and cytokine dosage were not performed in this case.
Differential diagnosis [23]
Neurological signs are variable and nonspecific; brain imaging helps confirm the diagnosis and rule out differential diagnoses:
- Cerebral venous thrombosis: Typically appears on imaging as a diffuse asymmetrical lesion, initially presenting with a high diffusion coefficient, which may later decrease.
- Encephalitis: Manifests with fever, leukocytosis, and unilateral brain involvement on brain imaging.
- Paraneoplastic or autoimmune encephalitis: The context is very suggestive, often occurring after vaccination or a viral infection, with the presence of antibodies or erythrocyte progenitors. It also causes unilateral involvement on CT and brain MRI.
- Cerebral edema in the post-critical phase or following prolonged hypoxemia.
- Brain tumor (lymphoma, glioma, brain metastasis): Clinical manifestations are chronic, and diagnosis is confirmed by biopsy; clinical and radiological signs are irreversible. Brain imaging shows a unilateral lesion.
- Progressive multifocal leukoencephalopathy: Shows hyperintensity on T2 FLAIR sequence, without enhancement after gadolinium injection.
- Osmotic demyelination syndrome due to a metabolic disorder.
Evolution and therapeutic management
Currently, no specific treatment exists for PRES; management primarily involves addressing the underlying cause to ensure symptom resolution. Seizures are treated with antiepileptics, and there is no specific protocol for treating this neurological sign. Normalization of blood pressure is crucial, with the goal of reducing blood pressure by 25% in the first few hours [24]. Continuous intravenous infusion of antihypertensive medication is required to achieve these goals [24]. A rapid drop in blood pressure can lead to cerebral ischemia. When PRES is caused by a specific treatment, temporarily discontinuing the treatment is recommended for the resolution of clinical signs. The progression is generally favorable, with the resolution of clinical and radiological signs within two weeks. In rare cases, clinical signs may persist due to immunosuppressive treatment that was not discontinued, as seen in kidney transplant recipients. In the absence of treatment, vasogenic edema can transform into cytotoxic edema [25].
In our case, the patient was initially treated with phenobarbital as a loading dose, along with sodium valproate and clobazam as maintenance treatment. Intravenous calcium channel blockers were administered to control blood pressure. Since our patient had lupus nephropathy, she was placed on nephroprotection to manage her blood pressure and we interrupted the maintenance anti-epileptic treatment after one month given the absence of recurrent seizures.
Ethical considerations
The authors confirm that written informed consent was obtained from the patient for the publication of this case report, including the accompanying clinical details and imaging findings. The patient was informed about the purpose of the publication, and consent was given with full understanding of the implications.
Additionally, the study adheres to the principles outlined in the Declaration of Helsinki and maintains compliance with institutional ethical guidelines. No identifying patient information has been disclosed in the manuscript to protect confidentiality.
For any further ethical inquiries regarding this case, the corresponding author may be contacted.
- Hinchey J, Chaves C, Appigani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. Available from: https://doi.org/10.1056/nejm199602223340803
- Wagih A, Mohsen L, Rayan MM, Hasan MM, Al-Sherif AH. Posterior reversible encephalopathy syndrome (PRES): restricted diffusion does not necessarily mean irreversibility. Pol J Radiol. 2015;80:210–6. Available from: https://doi.org/10.12659/pjr.893460
- Soo Y, Singhal AB, Leung T, Yu S, Mak H, Hao Q, et al. Reversible cerebral vasoconstriction syndrome with posterior leucoencephalopathy after oral contraceptive pills. Cephalalgia. 2010;30(1):42–5. Available from: https://doi.org/10.1111/j.1468-2982.2009.01868.x
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914-25. Available from: https://doi.org/10.1016/s1474-4422(15)00111-8
- Guyton AC, Hall JE. Cerebral blood flow, cerebrospinal fluid, and brain metabolism. In: Textbook of Medical Physiology. 12th ed. Philadelphia: Elsevier Saunders; 2011;743–750.
- Ugurel MS, Hayakawa M. Implications of post-gadolinium MRI results in 13 cases with posterior reversible encephalopathy syndrome. Eur J Radiol. 2005;53:441–449. Available from: https://doi.org/10.1016/j.ejrad.2004.05.015
- Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:254–58. Available from: https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.03.011
- Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry. 2010;81(7):773-777. Available from: https://doi.org/10.1136/jnnp.2009.189647
- Wu F, Liu L, Zhou H. Endothelial cell activation in central nervous system inflammation. J Leukoc Biol. 2017;101:1119–32. Available from: https://doi.org/10.1189/jlb.3ru0816-352rr
- Baizabal-Carvallo JF, Barragán-Campos HM, Padilla-Aranda HJ, Alonso-Juarez M, Estañol B, Cantú-Brito C, et al. Posterior reversible encephalopathy syndrome as a complication of acute lupus activity. Clin Neurol Neurosurg. 2009;111:359–63. Available from: https://doi.org/10.1016/j.clineuro.2008.11.017
- Kivity S, Agmon-Levin N, Zandman-Goddard G, Chapman J, Shoenfeld Y. Neuropsychiatric lupus: A mosaic of clinical presentations. BMC Med. 2015;13:43. Available from: https://doi.org/10.1186/s12916-015-0269-8
- Stock AD, Gelb S, Pasternak O, Ben-Zvi A, Putterman C. The blood-brain barrier and neuropsychiatric lupus: new perspectives in light of advances in understanding the neuroimmune interface. Autoimmun Rev. 2017;16:612–9. Available from: https://doi.org/10.1016/j.autrev.2017.04.008
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–9. Available from: https://doi.org/10.3174/ajnr.a0929
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219. Available from: https://doi.org/10.1093/eurheartj/eht151
- Abbott NJ, Mendonça LLF, Dolman DEM. The blood-brain barrier in systemic lupus erythematosus. Lupus. 2003;12:908–15. Available from: https://doi.org/10.1191/0961203303lu501oa
- Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–38. Available from: https://pubmed.ncbi.nlm.nih.gov/8669469/
- Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3759–69. Available from: https://doi.org/10.1002/art.23035
- Sato T, Fujii T, Yokoyama T, Fujita Y, Imura Y, Yukawa N, et al. Anti-U1 RNP antibodies in cerebrospinal fluid are associated with central neuropsychiatric manifestations in systemic lupus erythematosus and mixed connective tissue disease. Arthritis Rheum. 2010;62:3730–40. Available from: https://doi.org/10.1002/art.27700
- Nishimura K, Harigai M, Omori M, Sato E, Hara M. Blood-brain barrier damage as a risk factor for corticosteroid-induced psychiatric disorders in systemic lupus erythematosus. Psychoneuroendocrinology. 2008;33:395–403. Available from: https://doi.org/10.1016/j.psyneuen.2007.12.007
- Gelb S, Stock AD, Anzi S, Putterman C, Ben-Zvi A. Mechanisms of neuropsychiatric lupus: The relative roles of the blood-cerebrospinal fluid barrier versus blood-brain barrier. J Autoimmun. 2018;91:34–44. Available from: https://doi.org/10.1016/j.jaut.2018.03.001
- Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320–27. Available from: https://doi.org/10.3174/ajnr.a0549
- Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology. 2009;51:373–83. Available from: https://doi.org/10.1007/s00234-009-0504-0
- Lamy C, Oppenheim C, Meder JF, Mas JL. Neuroimaging in Posterior Reversible Encephalopathy Syndrome. J Neuroimaging. 2004;14:89-96. Available from: https://pubmed.ncbi.nlm.nih.gov/15095552/
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219. Available from: https://doi.org/10.1093/eurheartj/eht151
- Koob M, Dietmann JL. Encéphalopathie postérieure réversible. Presse Med. 2007;36:437-438. Available from: https://doi.org/10.1016/j.lpm.2007.02.005