More Information
Submitted: October 03, 2023 | Approved: October 19, 2023 | Published: October 20, 2023
How to cite this article: Hajji M, Mrad M, Bini I, Bahlous A, Khedher E, et al. Effects of Zinc Supplementation on Oxidative Stress in Patients Undergoing Maintenance Hemodialysis. J Clini Nephrol. 2023; 7: 092-096.
DOI: 10.29328/journal.jcn.1001116
Copyright License: © 2023 Hajji M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hemodialysis; Zn supplement; Copper to zinc ratio; Catalase activity; Homocysteine; Glutathione; Total bilirubin
Effects of Zinc Supplementation on Oxidative Stress in Patients Undergoing Maintenance Hemodialysis
Marwa Hajji1,2*, Mehdi Mrad1,3, Ines Bini4, Afef Bahlous1,3, Rania Khedher5, Karim Zouaghi1,5, Moncef Feki1,2 and Hayet Fellah1,2
1University of Tunis El Manar, Faculty of Medicine of Tunis, 1007 Tunis, Tunisia
2Laboratory of Biochemistry, LR99ES11, Rabta University Hospital, 1007 Tunis, Tunisia
3Laboratory of Biochemistry and Hormonology, Pasteur Institute, 1002 Tunis, Tunisia
4Laboratory of Biomolecules, venoms and Theranostic applications LR20IPT01, Pasteur Institute, 1002 Tunis, Tunisia
5Department of Nephrology, Rabta University Hospital, 1007 Tunis, Tunisia
*Address for Correspondence: Marwa Hajji, University of Tunis El Manar, Faculty of Medicine of Tunis, Laboratory of Biochemistry, Rabta University Hospital, 1007 Jebbari, Tunis, Tunisia, Email: [email protected]
Introduction: The aim of this study was to examine the effects of Zn supplementation on oxidative stress by evaluating changes in serum Copper (Cu) to Zinc (Zn) ratio, homocysteine (hCys), Glutathione (GSH), Total Bilirubin (TB) and Catalase (CAT) activity in hemodialysis patients.
Methods: Seventy-seven HD patients were enrolled in a multicenter simple-blind randomized clinical trial. Only 37 HD patients completed the study. They were randomly divided into two groups and supplemented with zinc sulfate (n = 17) or placebo (n = 20) for two months. Serum Zn and Cu were measured by atomic absorption spectrophotometry. Serum hCys was measured by immunology method, serum GSH and CAT activity were assessed by spectrophotometry method and TB was measured by colorimetric method. Determinations were performed before and after supplementation.
Findings: After zinc supplementation, serum Zn, serum GSH, and Serum Total Bilirubin (STB) significantly increased. Serum Cu to Zn ratio, serum hCys, and CAT activity significantly decreased in the Zn Zn-supplemented group.
Conclusion: Zinc supplementation increased serum antioxidant factors such as Zn, GSH, and bilirubin and decreased serum oxidative factors such as copper to zinc ratio, hCys, and decreased CAT activity. The study results suggest that zinc supplementation may be a useful tool for the improvement of oxidative stress in HD patients.
Patients undergoing maintenance hemodialysis exhibit a higher morbimortality due to cardiovascular disease [1] which can be attributed to many factors including oxidative stress [2,3]. Several studies have demonstrated zinc deficiency as a common problem in patients with HD [4]. It has been also shown that zinc deficiency plays considerable roles in the development and progression of CVD in these patients [5-7]. Zn is a trace element that acts as an antioxidant and anti-inflammatory agent [5,6]. Only a few studies have investigated the effects of zinc supplementation in HD patients on oxidative stress [8] also only a few studies have analyzed several SO parameters at the same time in the same group of patients. The impact on CAT activity and STB has never been analyzed.
The present study aimed to investigate the effects of Zn supplementation in HD patients on oxidative stress by evaluating changes in the levels of zinc, copper, homocysteine, reduced glutathione, catalase activity, and total bilirubin.
A multicenter randomized single-blind clinical trial was conducted among 77 HD patients (45 men and 32 women). The patients were recruited from five centers: Rabta Hospital Nephrology Department, Matri Hospital HD unit, Manouba HD Radial Center, Manouba HD Public Center as well and HD Udial Center. Causal nephropathies are distributed as follows: glomerulonephritis (27 patients), hypertensive nephropathy (16 patients), interstitial polynephritis (11 patients), polycystic disease (9 patients) and undetermined cause (14 patients).
The selection of patients included the following criteria: age over 18 years old, HD treatment for at least six months, HD performed three times per week (each for 4 hours) through a polysulfone membrane against a dialysis liquid containing the following ions: Na+: 138 mmol/L; K+: 2 mmol/L; Ca++: 1.5 mmol/L; Mg++: 0.5 mmol/L; Cl-: 109 mmol/L; CH3CO2-: 3 mmol/L; HCO3-: 35 mmol/L. The clearance of urea evaluated by the ratio Kt/V was 1.2. The exclusion criteria were infection, gastrointestinal and liver diseases, congestive heart failure, cancer, psychiatric illness, pregnancy, use of immunosuppressants, corticoids, estrogens, or contraceptives, as well as active smoking and alcoholism. The patients were randomly divided into two groups; 43 HD patients received one oral capsule containing 220 mg of Zn sulfate (100 mg of elemental Zn) and 34 HD patients received one oral capsule of similar appearance containing 220 mg of maltodextrin. Capsules were taken daily after dinner for 60 consecutive days. Randomization was performed while stratifying on gender, 5-year age class, and duration of HD. Predialysis blood samples were obtained from all patients after an overnight fast at inclusion (day 1)
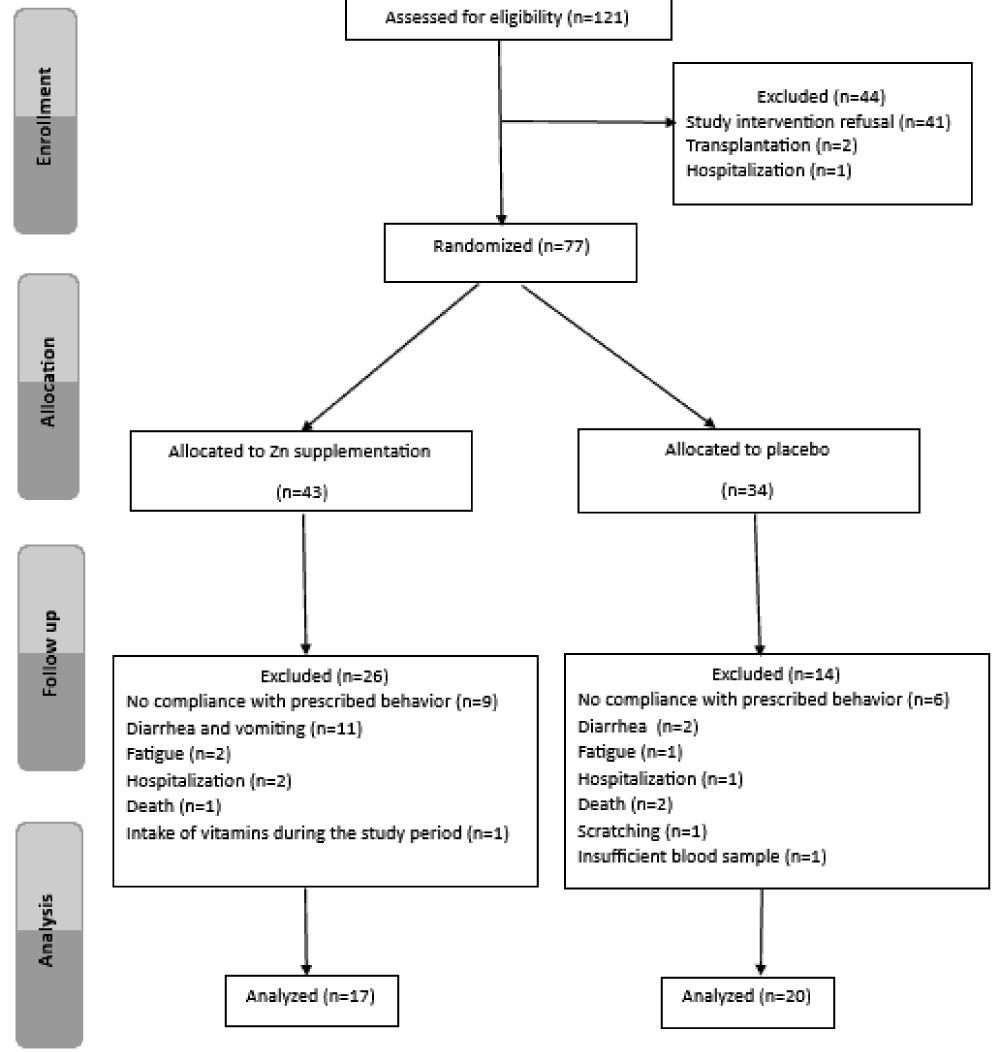
and after 60 days of supplementation (day 61). Blood samples of hCys were collected in tubes and placed on ice. Serum Zn and Cu concentrations were determined by atomic absorption spectrophotometry (Perkin Elmer, Waltham, USA). Serum hCys was measured by immunology method using the Architect CI8200 auto analyzer (Abbott Diagnostics, Chicago, USA), Serum GSH and CAT activity were assessed by spectrophotometry method. Serum TB was measured by colorimetric method. The study protocol was approved by the Ethics Committee of Rabta Hospital and all patients gave their informed and signed consent to participate in the study. A total of 37 HD patients (17 in the supplemented group and 20 in the placebo group) completed the trial (Figure 1).
Figure 1: Flow chart for the process of hemodialysis patients selection.
Statistical analysis
Statistical analyses were carried out using the software package SPSS 22.0 for Windows (SPSS Inc, Chicago, USA). Depending on the distribution of variables, data were reported as mean ± Standard Deviation (SD) or median (interquartile range). Between groups, comparisons were made using an independent t-test or Mann-Whitney test, as appropriate. Within groups, comparisons of variables before and after supplementation were performed using paired t-test or Wilcoxon rank test, as appropriate.
A p - value < 0.05 based on two-sided calculation was considered significant.
Baseline clinical and biochemical characteristics were comparable in Zn and placebo groups (Table 1).
| Table 1: Baseline clinical and biochemical characteristics in zinc-supplemented and placebo groups of hemodialysis patients. | |||
| Zinc group (n = 17) | Placebo group (n = 20) | p value | |
| Mean age, years | 52.9 ± 13.5 | 53.6 ± 15.8 | 0.89 |
| Male gender, % | 70.6 | 55.0 | 0.33 |
| Duration of HD, years | 6.65 ± 3.93 | 6.55 ± 3.73 | 0.93 |
| Body mass index, Kg/m2 | 25.2 ± 4.34 | 26.4 ± 5.74 | 0.53 |
| Serum creatinine, mmol/L | 967 ± 264 | 896 ± 361 | 0.50 |
| Serum zinc, µg/dL | 76.8 ± 16.6 | 81.2 ± 16.8 | 0.42 |
| Zinc deficiency *, % | 64.7 | 55.0 | 0.55 |
| Serum copper, µg/dL | 116 ± 33.2 | 117 ± 28.1 | 0.91 |
| Serum copper to zinc ratio | 1.52 ± 0.38 | 1.47 ± 0.35 | 0.62 |
| Serum hCys, µM/L | 21.07 ± 6.07 | 20.80 ± 8.04 | 0.90 |
| Serum GSH, U/mg of protein | 0.15 (0.23) | 0.12 (0.59) | 0.51 |
| CAT activity, U/mg of protein | 0.038 (0.15) | 0.045 (0.01) | 0.17 |
| Serum TB, mg/L | 4.76 ± 1.36 | 5.05 ± 1.76 | 0.62 |
| Values are expressed as mean ± SD or median (interquartile range); hCys: Homocysteine; GSH: Glutathione; CAT: Catalase; TB: Total Bilirubin; *, serum Zn < 80 µg/dL. | |||
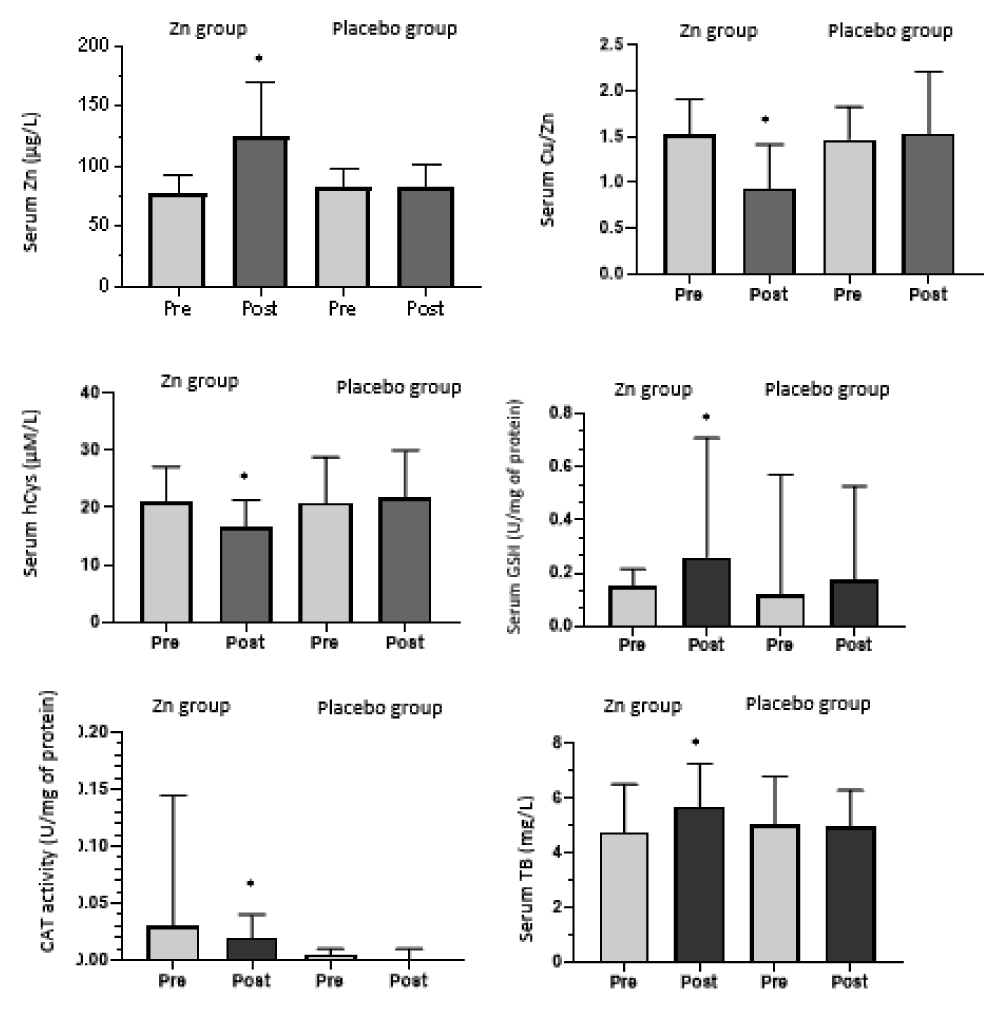
After two months of supplementation, serum Zn concentration increased (124 ± 46.4 µg/dl) (p = 0.002) and serum Cu to Zn ratio decreased significantly in the Zn supplemented group (0.93 ± 0.47) (p = 0.01) but remained unchanged in the placebo group. After supplementation, we found a significant increase in serum GSH (0.25 (0.72)) (p = 0.005) and TB (5.71 ± 1.58 mg/L) (p = 0.024). Serum hCys was significantly decreased (16.61 ± 4.69 µM/L) (p = 0.000) and also CAT activity (0 .021 (0.1)) (p = 0.02) and thus after zinc supplementation.
Figure 2 shows variations in zinc, Copper zinc ratio, homocysteine, glutathione, catalase activity, and total bilirubin under supplementation in zinc and placebo groups.
Figure 2: Variations in zinc, Copper to zinc ratio, homocysteine, glutathione, catalase activity and total bilirubin under supplementation in zinc and placebo groups.
Zinc deficiency is common in HD patients [9]. It has been reported in up to 50% of these patients [10-12]. The present study indicated that 59.5% of Tunisian HD patients have decreased Zn levels. Literature suggests that hypozincemia may be caused by Zn removal during HD treatment, reduced gastrointestinal absorption [8], low dietary intake, and increased urinary excretion [13] as well as protein restriction [14], increased expression of intracellular metallothioneins [15], multi-infections [10] and metabolic acidosis [16].
Zn supplementation in the present study significantly increased serum Zn and decreased serum Cu to Zn ratio, which is consistent with literature data [8]. The improvement in Zn status after Zn supplementation is expected and comprehensible. The competition between the two trace elements at the phases of intestinal absorption and cellular trafficking may explain the decrease in serum Cu after Zn supplementation. Indeed, Zn and Cu share the same enterocyte membranes’ transporters and intracellular trafficking proteins [17,18]. A high Cu reflects oxidative stress, increased inflammation, and immune dysfunction [18-20]. Thus, its reduction in HD patients is considered beneficial, which supports the usefulness of Zn supplementation in these patients.
The study also showed a decrease in serum hCys following Zn supplementation. hCys is a pro-oxidant component. It is a sulfur-containing amino acid metabolized through transsulfuration and transmethylation pathways. It is reported that hyperhomocysteinemia is an independent risk factor for cardiovascular mortality and morbidity in ESRD patients [21,22]. The beneficial effect of zinc supplementation on hCys levels could be explained by the fact that zinc contributes to the intestinal absorption of dietary folate. In fact, pteroylglutamatehydrolase, an enzyme responsible for the hydrolysis of dietary folate before its absorption, is zinc-dependent [23,24]. Zinc contributes also to the activity of BHMT (betaine-homocysteine methyltransferase), which is involved in the remethylation of homocysteine to methionine [14].
According to the results of our study, Zn supplementation increased serum GSH. GSH is a tripeptide composed of glutamate, cysteine, and glycine, that is synthesized from two sequential reactions catalyzed by glutamate-cysteine ligase and glutathione synthetase. The increase of serum GSH after supplementation could be explained in part by the fact that zinc induces the synthesis of glutathione by increasing the expression of glutamate cysteine ligase which catalyzes the formation of gamma-glutamylcysteine (γ-GC) from glutamate and cysteine [25].
To our knowledge, no previous studies have investigated the effect of zinc supplementation on CAT activity in HD patients. CAT is an important antioxidant enzyme. It allows the transformation of hydrogen peroxide into water and oxygen. Its activity requires the presence of iron. Its serum decrease following zinc supplementation could be linked to the decrease in the bioavailability of iron in relation to iron-zinc antagonism [26].
This study, to our knowledge, is the first that evaluates the effect of Zn supplementation on serum total bilirubin in HD patients. Low STB levels are associated with increased oxidative stress and may be a risk factor for Cardiovascular Diseases (CVD) [27]. High STB levels were associated with reduced risk of cardiovascular disease and mortality in dialysis patients [28].
Bilirubin has been known to have antioxidant properties [29,30], whether conjugated, unconjugated, free, or protein-bound [31]. Bilirubin directly inhibited NADPH oxidase activity and suppressed superoxide generation in vascular endothelial cells and renal tubular cells [32]. Bilirubin has been reported also to act as an oxidant scavenger [32]. After Zn supplementation, STB was significantly increased. Its serum increase following zinc supplementation could be explained by the fact that biliverdin reductase, an enzyme that catalyzes the reduction of biliverdin to bilirubin, is a Zn metalloprotein [33].
There are potential limitations to this study. First, our study was limited by a small sample, resulting in a low statistical power. In fact, a number of patients in both Zn and placebo groups dropped out from the trial for lack of compliance with the study design or for adverse health effects. Second, HD patients were supplemented without taking into account their baseline serum Zn concentrations. This might explain some side effects that occurred in some patients. Further, trials with large sample sizes in Zn-deficient patients are needed to confirm our results.
Zinc supplementation improved oxidative status in our HD patients. It increased serum anti-oxidative factors such as Zn, GSH, and bilirubin and decreased serum oxidative factors such as copper to zinc ratio, hCys, and CAT activity. The study results suggest that zinc supplementation may be a useful tool for stress oxidative therapeutic strategy in HD patients which may have implications for cardiovascular complications and propose monitoring of serum zinc concentration in this population.
The authors gratefully acknowledge the patients and administrative, nursing, and technical staff for their valuable help. Special thanks to “Vital Laboratory” for providing Zn sulfate and placebo.
- Wright J, Hutchison A. Cardiovascular disease in patients with chronic kidney disease. Vasc Health Risk Manag. 2009;5:713-22. doi: 10.2147/vhrm.s6206. Epub 2009 Sep 7. PMID: 19756163; PMCID: PMC2742701.
- Guo CH, Chen PC, Yeh MS, Hsiung DY, Wang CL. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin Biochem. 2011 Mar;44(4):275-80. doi: 10.1016/j.clinbiochem.2010.12.017. Epub 2011 Jan 9. PMID: 21223959.
- Rysz J, Franczyk B, Ławiński J, Gluba-Brzózka A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants (Basel). 2020 Nov 3;9(11):1079. doi: 10.3390/antiox9111079. PMID: 33153174; PMCID: PMC7693989.
- Takic M, Zekovic M, Terzic B, Stojsavljevic A, Mijuskovic M, Radjen S, Ristic-Medic D. Zinc Deficiency, Plasma Fatty Acid Profile and Desaturase Activities in Hemodialysis Patients: Is Supplementation Necessary? Front Nutr. 2021 Sep 23;8:700450. doi: 10.3389/fnut.2021.700450. PMID: 34631763; PMCID: PMC8496936.
- Sun JY, Jing MY, Wang JF, Zi NT, Fu LJ, Lu MQ, Pan L. Effect of zinc on biochemical parameters and changes in related gene expression assessed by cDNA microarrays in pituitary of growing rats. Nutrition. 2006 Feb;22(2):187-96. doi: 10.1016/j.nut.2005.07.007. Epub 2006 Jan 18. PMID: 16413754.
- Prasad AS. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp Gerontol. 2008 May;43(5):370-7. doi: 10.1016/j.exger.2007.10.013. Epub 2007 Nov 1. PMID: 18054190.
- Jern NA, VanBeber AD, Gorman MA, Weber CG, Liepa GU, Cochran CC. The effects of zinc supplementation on serum zinc concentration and protein catabolic rate in hemodialysis patients. J Ren Nutr. 2000 Jul;10(3):148-53. doi: 10.1053/jren.2000.7413. PMID: 10921536.
- Guo CH, Wang CL. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int J Med Sci. 2013;10(1):79-89. doi: 10.7150/ijms.5291. Epub 2012 Dec 22. PMID: 23289009; PMCID: PMC3534881.
- Elgenidy A, Amin MA, Awad AK, Husain-Syed F, Aly MG. Serum Zinc Levels in Chronic Kidney Disease Patients, Hemodialysis Patients, and Healthy Controls: Systematic Review and Meta-Analysis. J Ren Nutr. 2023 Jan;33(1):103-115. doi: 10.1053/j.jrn.2022.04.004. Epub 2022 Apr 25. PMID: 35472507.
- Rashidi AA, Salehi M, Piroozmand A, Sagheb MM. Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J Ren Nutr. 2009 Nov;19(6):475-8. doi: 10.1053/j.jrn.2009.04.005. Epub 2009 Jun 21. PMID: 19541504.
- Dashti-Khavidaki S, Khalili H, Vahedi SM, Lessan-Pezeshki M. Serum zinc concentrations in patients on maintenance hemodialysis and its relationship with anemia, parathyroid hormone concentrations and pruritus severity. Saudi J Kidney Dis Transpl. 2010 Jul;21(4):641-5. PMID: 20587866.
- Shiota J, Tagawa H, Izumi N, Higashikawa S, Kasahara H. Effect of zinc supplementation on bone formation in hemodialysis patients with normal or low turnover bone. Ren Fail. 2015 Feb;37(1):57-60. doi: 10.3109/0886022X.2014.959412. Epub 2014 Sep 10. PMID: 25207792.
- Bozalioğlu S, Ozkan Y, Turan M, Simşek B. Prevalence of zinc deficiency and immune response in short-term hemodialysis. J Trace Elem Med Biol. 2005;18(3):243-9. doi: 10.1016/j.jtemb.2005.01.003. PMID: 15966573.
- Kiziltas H, Ekin S, Erkoc R. Trace element status of chronic renal patients undergoing hemodialysis. Biol Trace Elem Res. 2008 Aug;124(2):103-9. doi: 10.1007/s12011-008-8135-6. Epub 2008 Apr 15. PMID: 18414814.
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151-72. doi: 10.1146/annurev.nutr.24.012003.132402. PMID: 15189117.
- Ghoreshi Z, Ahaley SK, Rasooli I. Effect of haemodialysis on trace elements in patients with acute and chronic renal failure. M J I R I. 2001; 14(4):329-331.
- Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007 Oct;98 Suppl 1:S29-35. doi: 10.1017/S0007114507832971. PMID: 17922955.
- Sudha R, Ponsuganthi K, Jones R. Serum zinc and copper levels in maintenance haemodialysis patients and its relationship with depression and anxiety. G J Med P H. 2015 ; 4.
- Karahan SC, Değer O, Orem A, Uçar F, Erem C, Alver A, Onder E. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin Chem Lab Med. 2001 Feb;39(2):109-15. doi: 10.1515/CCLM.2001.019. PMID: 11341743.
- Malavolta M, Giacconi R, Piacenza F, Santarelli L, Cipriano C, Costarelli L, Tesei S, Pierpaoli S, Basso A, Galeazzi R, Lattanzio F, Mocchegiani E. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology. 2010 Jun;11(3):309-19. doi: 10.1007/s10522-009-9251-1. Epub 2009 Oct 10. PMID: 19821050.
- Bostom AG, Shemin D, Verhoef P, Nadeau MR, Jacques PF, Selhub J, Dworkin L, Rosenberg IH. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. A prospective study. Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2554-8. doi: 10.1161/01.atv.17.11.2554. PMID: 9409227.
- Moustapha A, Naso A, Nahlawi M, Gupta A, Arheart KL, Jacobsen DW, Robinson K, Dennis VW. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation. 1998 Jan 20;97(2):138-41. doi: 10.1161/01.cir.97.2.138. Erratum in: Circulation 1998 Feb 24;97(7):711. PMID: 9445164.
- Williams RB, Mills CF, Davidson RJ. Relationships between zinc deficiency and folic acid status of the rat. Proc Nutr Soc. 1973 May;32(1):2A-3A. PMID: 4760784.
- Tamura T, Shane B, Baer MT, King JC, Margen S, Stokstad EL. Absorption of mono- and polyglutamyl folates in zinc-depleted man. Am J Clin Nutr. 1978 Nov;31(11):1984-7. doi: 10.1093/ajcn/31.11.1984. PMID: 717270.
- Samman S, Foster M. Zinc and cardio-metabolic health in type 2 diabetes mellitus. Vitam Trace Elem. 2013; 2:116.
- Mariani E, Mangialasche F, Feliziani FT, Cecchetti R, Malavolta M, Bastiani P, Baglioni M, Dedoussis G, Fulop T, Herbein G, Jajte J, Monti D, Rink L, Mocchegiani E, Mecocci P. Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Exp Gerontol. 2008 May;43(5):445-51. doi: 10.1016/j.exger.2007.10.012. Epub 2007 Nov 1. PMID: 18078731.
- Boon AC, Bulmer AC, Coombes JS, Fassett RG. Circulating bilirubin and defense against kidney disease and cardiovascular mortality: mechanisms contributing to protection in clinical investigations. Am J Physiol Renal Physiol. 2014 Jul 15;307(2):F123-36. doi: 10.1152/ajprenal.00039.2014. Epub 2014 Apr 23. PMID: 24761005.
- Wagner KH, Wallner M, Mölzer C, Gazzin S, Bulmer AC, Tiribelli C, Vitek L. Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci (Lond). 2015 Jul;129(1):1-25. doi: 10.1042/CS20140566. PMID: 25881719.
- Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004 Oct;66(4):773-9. doi: 10.1124/mol.104.002832. Epub 2004 Jul 21. PMID: 15269289.
- Vítek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012 Apr 3;3:55. doi: 10.3389/fphar.2012.00055. PMID: 22493581; PMCID: PMC3318228.
- Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000 Nov;46(11):1723-7. PMID: 11067805.
- Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal. 2016 Jul 20;25(3):119-46. doi: 10.1089/ars.2016.6665. Epub 2016 Apr 26. PMID: 26906267; PMCID: PMC4948213.
- Maines MD, Polevoda BV, Huang TJ, McCoubrey WK Jr. Human biliverdin IXalpha reductase is a zinc-metalloprotein. Characterization of purified and Escherichia coli expressed enzymes. Eur J Biochem. 1996 Jan 15;235(1-2):372-81. doi: 10.1111/j.1432-1033.1996.00372.x. PMID: 8631357.