More Information
Submitted: September 19, 2023 | Approved: October 09, 2023 | Published: October 10, 2023
How to cite this article: Mahmoud NB, Hamouda M, Maatoug J, Salem MB, Salah MB, et al. Clinical and Epidemiological Profile of Reversible Acute Kidney Injury with Full Recovery: Experience of a Nephrology Department. J Clini Nephrol. 2023; 7: 078-084.
DOI: 10.29328/journal.jcn.1001114
Copyright License: © 2023 Mahmoud NB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Maghreb; Epidemiology; Acute kidney injury; Recovery of normal renal function
Clinical and Epidemiological Profile of Reversible Acute Kidney Injury with Full Recovery: Experience of a Nephrology Department
Nouha Ben Mahmoud1*, Mouna Hamouda2, Jihene Maatoug3, Meriem Ben Salem1, Manel Ben Salah1, Ahmed Letaief4, Sabra Aloui5 and Habib Skhiri5
1Doctor, Nephrology Dialysis and Transplantation Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia
2Assistant Professor, Nephrology Dialysis and Transplantation Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia
3Professor, Epidemiology Department, Farhat Hached University Hospital, Sousse, Tunisia
4Assistant Professor, Nephrology Dialysis and Transplantation Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia
5Professor, Nephrology Dialysis and Transplantation Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia
*Address for Correspondence: Nouha Ben Mahmoud, Doctor, Nephrology Dialysis and Transplantation Department, Fattouma Bourguiba University Hospital, Monastir, Tunisia, Email: [email protected]
Purpose: Acute kidney injury (AKI) is a real public health problem due to its severity and gravity. In a 2013 meta-analysis, Susantitaphong, et al. estimated the incidence of AKI worldwide at between 10% and 20%. In the latter study, no African studies were included, given the lack of data in the literature. Our objective was to identify the clinical and paraclinical epidemiological characteristics of patients with AKI.
Patients and methods: We conducted a retrospective study including patients who had AKI with recovery of normal renal function hospitalized in a nephrology service between 2002 and 2015.
Results: Our population consisted of 107 men and 107 women with a median age of 61 years (IQR 43-73.25) of which 42.1% were multitargeted. Functional AKI represented the predominant mechanism of AKI retained in our study with a rate of 53.2% with dehydration as the main etiology for 108 patients (50.46%). Organic cause was retained in 38.8% of patients, with acute tubular necrosis (ATN) as the most frequent etiology (37.35%). Kidney disease improving global outcomes (KDIGO) stage 3 was the stage retained for 115 patients included in our series, 31 of whom required extra renal purification. During their hospitalization, 78.5% of the patients presented a persistent AKI (duration of the episode > 7 days). A glomerular filtration rate (GFR) lower than 60 ml/min/1.73 m² at discharge was found in 119 patients and 10 patients had a GFR higher than 90 ml/min/1.73 m². After 3 months from discharge, 77.5% of patients had a GFR between 60 and 90 ml/min/1.73 m².
Conclusion: Our results give us an idea of the epidemiological and clinical characteristics of patients who have had acute renal failure with recovery of normal renal function and enable us to better recognize patients at risk in order to avoid such complications. AKI remains a major issue and knowing its epidemiological and clinical characteristics will allow its prevention.
Acute kidney injury (AKI) is a global health problem that occurs in hospital wards, whether medical or surgical. Its prevalence is estimated between 1 and 25% according to studies [1] i.e. 13.3 million people per year [2]. It is a diagnostic and therapeutic emergency designating a syndrome characterized by a rapid decline in the excretory function of the kidney with accumulation of products of nitrogen metabolism, such as creatinine and urea, resulting in hydroelectrolytic and acid-base disorders [2].
It is a clinico-biological syndrome encompassing the whole spectrum of acute renal failure leading to avoidable deaths making intervention urgent and fundamental.
In Tunisia, a developing country, epidemiological data on AKI in a nephrology department are rare or even non-existent even in Africa [3].
Considering the lack of national and Maghreb level data concerning AKI in a nephrology department and especially data concerning patients having had an episode of AKI with the recovery of a normal renal function, we decided to carry out our work in order to recognize the patients at risk and thus improve their management.
Our objective was to describe the epidemiological, clinical, and biological characteristics of patients who had an episode of AKI with a return to normal renal function.
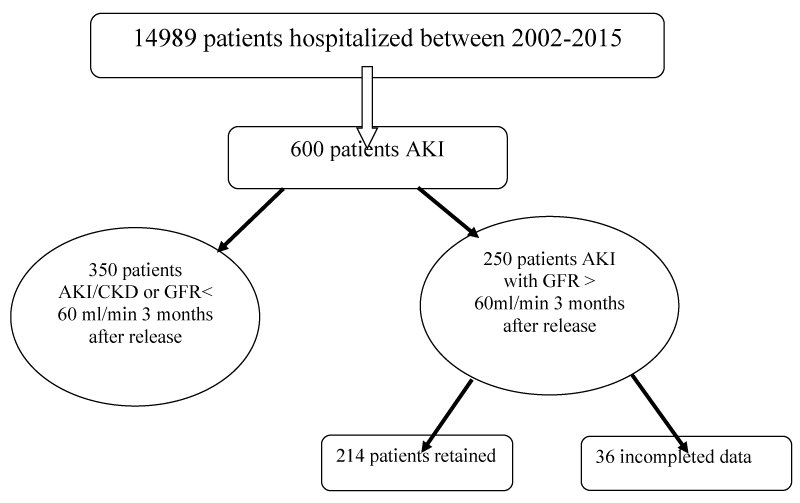
This is a retrospective and descriptive study that included patients hospitalized in a Nephrology Department having coding AKI between the month of January 2002 and the month of December 2015 (Figure 1).
Figure 1: Characteristics of the study population: Study flowchart.
Study population
The patients included were those whose age was ≥15 years because, in our department, we hospitalize patients over the age of 15, as they are no longer considered children, and those who had presented an AKI and recovered normal renal function.
The presence of AKI was defined according to the KDIGO criteria, which are:
- A creatinine increase of 3 mg/L (26.5 μmol/L) within 48 hours or
- An increase in creatinine of more than 1.5 times within 7 days compared with baseline creatinine. We included all types and etiologies of AKI.
Patients not included were those with known chronic kidney disease (CKD), renal transplant patients, chronic hemodialysis or peritoneal dialysis patients, and patients who died during hospitalization.
Anamnestic, clinical, and therapeutic survey data were collected according to a predefined form.
Baseline creatinine levels were those of the check-ups performed before hospitalization if available.
The creatinine values retained were the highest values during the hospitalization in order to determine the severity of the AKI according to the KDIGO staging [4].
The pathophysiological parameters found by questioning the patients, as well as the clinical and paraclinical signs, were used to classify the etiology of AKI as pre-renal, renal, or postrenal AKI.
A renal biopsy was performed when indicated after the collection of clinical and paraclinical data.
Age, sex, patient history, and department of origin were collected, with the calculation of the Charlson Comorbidity Index (CCI).
Data from the clinical examination on admission and from the biological tests performed were taken from the patient’s medical records.
The glomerular filtration rate was calculated by the Modification of Diet in Renal Disease (MDRD) formula [5].
Functional AKI was defined as an AKI secondary to hypovolemia, cardiorenal syndrome, and nephrotic syndrome.
Recovery of normal renal function was defined as a return to baseline renal function and/or a GFR > 60ml/min/1.73 m² at discharge or after 3 months.
CKD was defined as a glomerular filtration rate below 60 ml/min/1.73 m² for more than 3 months [4].
Elderly patients were defined as patients older than 65 years.
Treatment was either symptomatic based mainly on extrarenal purification with correction of hydrolytic disorders or curative with rehydration, antibiotic therapy, or urinary drainage.
Data analysis
The results were expressed by their means (+/- standard deviation) with [minimum and maximum]. Otherwise, variables were expressed by their median and (interquartile range IQR1, IQR3). Categorical variables were expressed as simple frequency and relative frequency (percentage). To compare the means of independent variables, a Student’s t - test for unpaired variables was used. To study the association of risk factors and the occurrence of morbidity, the Chi-square (Chi-2) test was used to compare percentages. In all statistical tests, the 5% threshold was used for statistical significance. All tests were performed with SPSS 23 software.
Ethical considerations
When we carried out our work in 2021, there was no ethics committee available to give us its opinion in our region and personal consent was obtained from the patients themselves, or their family members if they were deceased.
Our population included 107 men and 107 women with a median age of 61 years, ranging from 15 to 93 years. Elderly patients represented 43.5% of our population. The main history of the patients is summarized in Table 1. There was a positive correlation between age and the number of patient histories (r = 0.417; p < 10-3).
| Table 1: Main patient comorbid conditions. | ||
| Comorbid conditions | n | % |
| Hypertension (HTN) | 80 | 37.4 |
| Diabetes | 69 | 32.2 |
| Cardiopathy | 17 | 7.9 |
| Ischemic vascular stroke (IVS) | 21 | 9.8 |
| Chronic obstructive pulmonary disease (COPD) | 11 | 5.1 |
| Urologic | 8 | 3.7 |
| Neoplasia | 7 | 3.2 |
| n: number of patients; %: Percentage of patients | ||
The distribution of CCI scores was as follows: score 0: 52%, score 1-4: 44%, score 5-6: 3% score 7-8: 1%. Using the age-adjusted ICC, 109 patients had an index between 1 and 4. One hundred and twenty-four patients or 57.4% were from the emergency department.
Clinical presentation
Of the patients included in our study, 31 patients had presented with acute complications such as acute lung edema, life-threatening hyperkalemia, and severe metabolic acidosis.
The circumstances of discovery and reasons for hospitalization were:
- A systematic discovery in front of the observation of an increase in creatinine in 50 patients or 23.3% of cases.
- A change in diuresis such as oliguria in 117 patients (54.6% of cases) and anuria in 11 patients.
- Hematuria and back pain in 9 and 13 patients respectively.
- The appearance of signs of acute uraemia in one patient and the appearance of other symptoms in 15 patients such as dyspnoea, oedema, etc.
The number of patients with general signs was 132 with an alteration of the general state in 24 patients (18.3%) and asthenia alone was described by 20 patients (15%). Elevated blood pressure on admission was found in 71 patients. All patients included in our study had a renal ultrasound and it was normal for 96.7% of the population. Hypocalcemia was noted in 58% of patients. The results of the various tests performed at the time of admission of the patients are summarized in Table 2.
| Table 2: Results of biological tests at admission. | ||||
| Variables | n | Median | Min | Max |
| Urea (mmol/l) | 214 | 24.7 | 5.3 | 91 |
| Creatinine (µmol/l) | 214 | 335.5 | 112 | 3057 |
| Calcium (mmol/l) | 202 | 2.2 | 1.43 | 4.79 |
| Sodium (mmol/l) | 214 | 135 | 112 | 178 |
| Potassium (mmol/l) | 214 | 4,38 | 2.27 | 9.7 |
| Phosphorus (mmol/l) | 193 | 2 | 0.57 | 5.56 |
| Albumin (g/l) | 96 | 28.65 | 7 | 47.4 |
| Hemoglobin (g/dl) | 214 | 11.15 | 4.1 | 19.3 |
| n: number of patients; Min: Minimum; Max: Maximum | ||||
Etiological presentation
AKI was of pre-renal mechanism in 53% of patients, renal in 39%, and post-renal in 8%. Patients with pre-renal AKI were older (p < 10-3) than patients with renal AKI. The length of stay of patients with renal AKI was longer (p = 0.03) than other patients.
KDIGO Stage 3 was the majority stage as it was noted in 115 patients (53.7%). The remaining patients were classified as stage 2: 49 patients (22.9%) and stage 1: 50 patients (23.4%). Men had more severe AKI than women according to KDIGO stages and this difference was statistically significant (p = 0.019).
The majority of the modified CCI scores represented 1 to 4 in 52%, 51%, and 50.4% of patients among the different KDIGO stages respectively.
Renal biopsy was indicated and performed for 25 patients. The indications were isolated hematuria and unexplained AKI for 9 and 7 patients respectively.
The kidney biopsy was contributory in 23 patients. The lesions identified were:
- Tubulo interstitial: acute interstitial nephritis (AIN) in 6 patients and isolated acute tubular necrosis (ATN) in 3 patients.
- Glomerular: 6 patients had extracapillary glomerulonephritis (ECG), 3 patients had acute glomerulonephritis (AGN) lesions, 2 patients had minimal glomerular damage (MGD) associated with ATN, and 1 patient had evidence of isolated amyloidosis.
Pathophysiologic types
Based on the findings of the interrogation, clinical examination, and paraclinical examinations, bilateral obstructive lithiasis was the most frequent etiology found in 9 patients (4.2%). Bladder tumors and prostate adenomas were found in 5 and 3 patients respectively.
The most frequent etiology of functional AKI was dehydration with hypovolemia, which was found in 108 patients (94.7%). Vomiting and diarrhea were the most frequent causes of dehydration (79 patients), followed by diuretic intake (25 patients) and sepsis in 4 patients. During the summer period, from June 1st to August 31st, 36 patients were hospitalized for dehydration, while the number was 27 patients during the winter period. A cardiorenal syndrome secondary to a decompensation of heart failure was noted in 3 patients. One patient had AKI secondary to a nephrotic syndrome and 2 other patients had AKI secondary to an alteration of intra-renal hemodynamics by taking a renin-angiotensin system inhibitor. AKI secondary to an intra-renal mechanism was found in 83 patients (38.8%), the etiologies of which were dominated by NTA in 30 patients, i.e. 36.1% of cases with organic AKI. NSAID intake was incriminated in the genesis of AKI in 11 patients.
Management
Hemodialysis was indicated in 31 patients; it was prophylactic in the presence of anuria of more than 48 hours and urea of more than 40 in one patient. It was curative in 30 patients.
- Threatening hyperkalemia was the indication for 19 patients.
- Pulmonary oedema (PO) was the indication for 6 patients.
- Metabolic acidosis was the indication for 5 patients.
The total number of dialysis sessions performed was 70 sessions with a minimum of one session and a maximum of 8 sessions performed for two patients. The comparison of patient characteristics according to the need or not of extra renal purification is summarized in Table 3.
| Table 3: Comparison of patient characteristics according to the need for extrarenal treatment. | ||||
| EET- | EET+ | p | ||
| Effectives | n (%) | 183 (85.6) | 31 (14.4) | |
| Age (years) | Median (Q1-Q3) | 62 (43.2-74) | 54 (40-70) | 0.238 |
| Sex | M/F | 91/93 | 16/15 | 0.824 |
| DS (days) | Median (Q1-Q3) | 8 (5-12) | 9 (6-17) | 0.150 |
| AKI mechanism | 0.003* | |||
| Pre renal | n (%) | 106 (57.9) | 8 (25.8) | |
| Renal | n (%) | 65 (35.5) | 18 (58) | |
| Post renal | n (%) | 12 (6.6) | 5 (16.2) | |
| Hb (g/dl) | 0.066 | |||
| < 8 | n (%) | 16 (8.7) | 7 (2.3) | |
| 8 - 12 | n (%) | 97 (53) | 13 (41.2) | |
| < 12 | n (%) | 71 (38.3) | 11 (35.5) | |
| *p ≤ 0.05, n: number; Q1: 1st quartile; Q3: 3rd quartile; Hb: Hemoglobin; M: Male; F: Female; EET: Extrarenal Treatment | ||||
The main etiopathogenic treatments consisted of rehydration in 61.4% of cases, cessation of nephrotoxic treatment in 6% of cases, and corticosteroid therapy in 6% of cases.
Short-term evolution
A transient AKI (T-AKI), i.e. a duration of the episode of less than 7 days was noted in 46 patients. There was no significant difference in the characteristics of patients with T-AKI compared to those with persistent AKI (P-AKI).
The median length of hospitalization was 9 days with a minimum of 1 day and a maximum of 120 days.
An eGFR below 60 ml/min/1.73 m² at discharge was found in 119 patients and 10 patients had an eGFR above 90ml/min/1.73 m². After 3 months from discharge, 77.5% of patients had an eGFR between 60 and 90 ml/min/1.73 m². The comparison of patient characteristics by an eGFR at 3 months is shown in Table 4.
| Table 4: Comparison of patient characteristics by GFR at 3 months. | ||||
| Variables | 60-89 ml/min | ≥ 90 ml/min | p | |
| Effectives | n (%) | 166 (77.5%) | 48 (22.5%) | |
| Age (years) | Med (IQR1-Q3) | 64 (46.75-74) | 50 (36.5-65.75) | 0.012* |
| Sex | M/F | 75/91 | 32/16 | 0.009* |
| Comorbidities | n (%) | 75 (45.2) | 11 (22.9) | 0.006* |
| Length of stay (days) | Med (IQR1-IQR3) | 9 (5-13) | 9 (5-12.75) | 0.626 |
| Number of comorbid conditions | Med (IQR1-IQR3) | 1 (0-2) | 1 (0-1) | 0.004* |
| CCI modified | Med (IQR1-IQR3) | 4 (2-5) | 2 (0-4) | < 10-3* |
| CCI | Med (IQR1-IQR3) | 1 (0-2) | 0 (0-1) | 0.002* |
| KDIGO classification | 0.109 | |||
| Stage 1 | n (%) | 43 (25.9) | 7 (14.6) | |
| Stage 2 | n (%) | 40 (24.1) | 9 (18.7) | |
| Stage 3 | n (%) | 83 (50) | 32 (66.7) | |
| Hypertension | n (%) | 70 (42.2) | 10 (20.8) | 0.007* |
| Diabetes | n (%) | 60 (36.1) | 9 (18.7) | 0.023* |
| EET | n (%) | 22 (13.2) | 9 (18.7) | 0.341 |
| Elderly persons | 0.009* | |||
| < 65 years | n (%) | 86 (51.8) | 35 (72.9) | |
| ≥ 65 years | n (%) | 80 (48.2) | 13 (27.1) | |
| *p ≤ 0.05, n: number of cases; %: Percentage; M: Male; F: Female; Med: Median; IQR1: 1stquartile; IQR3: 3rd quartile; EET: Extrarenal Treatment; CCI: Charlson Comorbidity Index | ||||
Long-term evolution
After discharge, 34 patients were rehospitalized, i.e. 15.9% of the study population. Of these, 6 patients were hospitalized in urology and 6 had presented a fracture indicating their hospitalization in orthopedics. A recurrence of AKI was noted in 4.3% of the patients included. Infection requiring hospitalization in infectious diseases was noted in 5 patients.
The long-term evaluation involved only 151 patients, 5 of whom progressed to CKD with a mean delay of 155.98 ± 7.2 months.
In the long term, death occurred in 26 (16.5%) patients with a mean delay of 103.6 ± 6.63 months.
Our results allowed us to affirm that in our country, AKI remains a serious, severe condition that requires adequate management to prevent its consequences.
Our population was composed of 107 men and 107 women with a median age of 61 years (IQR 43-73.25) of which 42.1% had multiple comorbid conditions. Functional AKI was the predominant mechanism of AKI in our study with a rate of 53.2% with dehydration as the main etiology for 108 patients (50.46%).
The proportion of patients with AKI in our study is similar to that found in the literature. Long, et al. [6] estimated, in a study done between 1993 and 2013 in Iceland, that the incidence of AKI was 25.8 per thousand admissions/year. Kaul, et al. [7], in their study done in 2012 in a nephrology department in India had found an incidence of 2.5% with 45% of the patients regaining normal renal function.
The differences in incidence can be explained by the variability of definitions used by each team, some used the definition according to RIFLE, others AKIN and the most recent ones opted for the 2012 KDIGO definition with its classification [8,9]. The accessibility to the health system contributes to explaining the differences found, in developed countries, an annual consultation is mandatory by the attending physician allowing the discovery and rapid management of AKI which would explain a much higher incidence in high-income countries than in developing countries where access to care is limited to life-threatening emergencies.
In our study, the median age of patients with an episode of AKI was 61 years, which was largely consistent with studies from developed countries, with a sex ratio of 1H/1F [10,11]. The mean age of the different series in the literature is around 62 years with a clear male predominance ranging from 50% to 98% depending on the series [12,13].
As described in several studies, the incidence of AKI increases with age, as in our study [10,11]. In Tunisia, few studies had the objective of studying the epidemiology of patients in a nephrology department, we cite for example the work of Ariba, et al. [8] found that the average age of patients hospitalized in the internal medicine department of the military hospital of Tunis was 59.8 years, with a male predominance (sex ratio 1.76), but this work included all patients who had an AKI during their stay in the department, regardless of the recovery or persistence of the AKI.
The patients included in our series were multitargeted in 42.3% of cases with 81 hypertensive patients and 32.1% were diabetic with a median Charlson Comorbidity Index of 0 with a maximum of 7. In the literature, the most common histories were hypertension, diabetes, and cardiovascular pathologies [11,14] as described in the series of Ikizler, et al. [13] and Hatakeyama, et al. [12].
Our results are different from those found in several studies where patients had a mean Charlson Comorbidity Index of 5, they had a much higher history of malignant diseases than what was found in our study [15-18].
The results of the clinical data of our patients were different from the results found by Ariba, et al. [8] and Lengani, et al. [9] and these differences can be explained by the fact that they included all the patients who had an AKI and the particularity of our service is that it has an intensive care unit where patients with visceral failures are present contrary to several series carried out in nephrology services where they only hospitalized patients who were not serious.
In our study, the medians of creatinine and urea were respectively 335 µmoles/l and 24.7 mmoles/l, which was close to the biological results found by Ariba, et al. in 2013 [8]. Hypocalcemia was found in 58% of the patients, 38 of whom had false hypocalcemia, a result similar to that found by Lengani, et al. [9].
Although hypocalcemia was described by several authors as a presumptive criterion for CKD [19], more than half of the patients in our series had hypocalcemia, which was transient and sometimes related to an etiology of AKI such as rhabdomyolysis. This disorder was rapidly corrected after the restoration of normal renal function.
Several mechanisms have been investigated as being involved in the genesis of hypocalcemia in AKI, such as decreased synthesis of 1,25 OH vitamin D, decreased digestive absorption of calcium, decreased renal reabsorption of calcium, and decreased bone calcium release [19].
In our study, 115 patients were classified as KDIGO stage 3 and the most common etiology was dehydration (109 patients), i.e. pre-renal AKI in 50.6% of cases. This result was comparable to that found by Fernandes, et al. [20] (77% of patients with stage 3 KDIGO), but this was not the case with the studies carried out by Ikizler, et al. [13], Heung, et al. [15] and Teo, et al. [21] who found a clear predominance of stage 1 KDIGO.
Other studies found different results because they used different classifications to stage the severity of AKI; RIFLE classification for Ruiz-criado, et al. [18] and Arias-Cabrales, et al. [14] and AKIN classification for Jones, et al. [22]. Pre-renal etiology was most frequent in the series of Teo, et al. [21], Fernandes, et al. [20] and Ruiz-Criado, et al. [18].
In our study, a kidney biopsy was performed on 25 patients (11.7%). In the series of Ariba, et al. [8], kidney biopsy was performed in 12 cases; in front of unexplained AKI in 4 cases, rapidly progressive glomerulonephritis in 4 cases, and abundant proteinuria and/or hematuria in 4 cases. In the study by Lengani, et al. [9], it was performed in only 2 patients.
This can be explained by the fact that a small number of patients presented an indication for kidney biopsy and that the countries of sub-Saharan Africa have a poor technical platform and not easy access to this technique.
In our series, extra renal replacement therapy (EET) was indicated in 31 patients, i.e. 14.4% of the population studied, which is in line with the results found by Pannu, et al. [17] (7.6%), Ruiz-Criado, et al. [18] (13.7%) and Arias Cabrales, et al. [14] (10%).
However, significant variability in the use of EET has been noted in the literature ranging from 0.8 and 90% of patients [9,16,20,21,23-26].
Despite this discordance, there are situations in which it is essential to start an EET without delay. These absolute indications are generally well accepted by all teams. These are threatening hyperkalemia with electrical signs, severe acidosis with pH < 7.1, and PO unresponsive to high-dose diuretics [27]. According to Fernandes, et al. [20], metabolic acidosis and PO refractory to high-dose diuretics were the most frequent indications in his series with 39% and 44.6% respectively.
The majority of patients included in our study had recovered normal renal function at 3 months and not at discharge, these results were not comparable with those found in the literature. Since the majority of studies had a maximum follow-up of 90 days after hospital discharge [13,21,23].
AKI is a serious and severe phenomenon that is avoidable in some cases, particularly frequent in our country. In Tunisia, epidemiological data are scarce concerning this phenomenon, hence the importance of carrying out other multicentric and prospective studies to assess its real importance. Prevention and early detection can avoid this complication which still keeps an important morbi-mortality. The interest of our study was to recognize the epidemiological and clinical characteristics of patients who had an AKI with recovery of normal renal function, in order to determine the risk factors of this major event. Prevention perspectives: sensitize general practitioners and other specialties to the importance of assessing renal function in patients at risk for AKI because of their history or the procedure or treatment that puts them at risk for developing AKI.
- Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet. 2013 Jul 13;382(9887):170-9. doi: 10.1016/S0140-6736(13)60647-9. Epub 2013 May 31. PMID: 23727171.
- Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015 Jun 27;385(9987):2616-43. doi: 10.1016/S0140-6736(15)60126-X. Epub 2015 Mar 13. PMID: 25777661.
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013 Sep;8(9):1482-93. doi: 10.2215/CJN.00710113. Epub 2013 Jun 6. Erratum in: Clin J Am Soc Nephrol. 2014 Jun 6;9(6):1148. PMID: 23744003; PMCID: PMC3805065.
- Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray P, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Notice. Kidney International Supplements. 2012; 2(1): 1. https://doi.org/10.1038/kisup.2012.1
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247-54. doi: 10.7326/0003-4819-145-4-200608150-00004. Erratum in: Ann Intern Med. 2008 Oct 7;149(7):519. Erratum in: Ann Intern Med. 2021 Apr;174(4):584. PMID: 16908915.
- Long TE, Sigurdsson MI, Sigurdsson GH, Indridason OS. Improved long-term survival and renal recovery after acute kidney injury in hospitalized patients: A 20 year experience. Nephrology (Carlton). 2016 Dec;21(12):1027-1033. doi: 10.1111/nep.12698. PMID: 26660951.
- Kaul A, Sharma RK, Tripathi R, Suresh KJ, Bhatt S, Prasad N. Spectrum of community-acquired acute kidney injury in India: a retrospective study. Saudi J Kidney Dis Transpl. 2012 May;23(3):619-28. PMID: 22569459.
- Ariba Y. Acute renal failure in an internal medicine department: epidemiology, etiology and prognosis. About 160 cases [Retrospective study]. Faculty of Medicine of Tunis. 2013.
- Lengani A, Kargougou D, Fogazzi GB, Laville M. L'insuffisance rénale aiguë au Burkina Faso [Acute renal failure in Burkina Faso]. Nephrol Ther. 2010 Feb;6(1):28-34. French. doi: 10.1016/j.nephro.2009.07.013. PMID: 19836324.
- Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009 Jun;53(6):961-73. doi: 10.1053/j.ajkd.2008.11.034. Epub 2009 Apr 5. PMID: 19346042; PMCID: PMC2726041.
- See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, Toussaint ND, Bellomo R. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019 Jan;95(1):160-172. doi: 10.1016/j.kint.2018.08.036. Epub 2018 Nov 23. PMID: 30473140.
- Hatakeyama Y, Horino T, Nagata K, Matsumoto T, Terada Y, Okuhara Y. Transition from acute kidney injury to chronic kidney disease: a single-centre cohort study. Clin Exp Nephrol. 2018 Dec;22(6):1281-1293. doi: 10.1007/s10157-018-1571-5. Epub 2018 Apr 9. PMID: 29633059.
- Ikizler TA, Parikh CR, Himmelfarb J, Chinchilli VM, Liu KD, Coca SG, Garg AX, Hsu CY, Siew ED, Wurfel MM, Ware LB, Faulkner GB, Tan TC, Kaufman JS, Kimmel PL, Go AS; ASSESS-AKI Study Investigators. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021 Feb;99(2):456-465. doi: 10.1016/j.kint.2020.06.032. Epub 2020 Jul 22. PMID: 32707221; PMCID: PMC7374148.
- Arias-Cabrales C, Rodríguez E, Bermejo S, Sierra A, Burballa C, Barrios C, Soler MJ, Pascual J. Short- and long-term outcomes after non-severe acute kidney injury. Clin Exp Nephrol. 2018 Feb;22(1):61-67. doi: 10.1007/s10157-017-1420-y. Epub 2017 May 27. PMID: 28551821.
- Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, Powe NR, Pavkov ME, Williams DE, Saran R, Shahinian VB; Centers for Disease Control and Prevention CKD Surveillance Team. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2016 May;67(5):742-52. doi: 10.1053/j.ajkd.2015.10.019. Epub 2015 Dec 12. PMID: 26690912; PMCID: PMC6837804.
- Hickson LJ, Chaudhary S, Williams AW, Dillon JJ, Norby SM, Gregoire JR, Albright RC Jr, McCarthy JT, Thorsteinsdottir B, Rule AD. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am J Kidney Dis. 2015 Apr;65(4):592-602. doi: 10.1053/j.ajkd.2014.10.015. Epub 2014 Nov 5. PMID: 25500361; PMCID: PMC4630340.
- Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013 Feb;8(2):194-202. doi: 10.2215/CJN.06480612. Epub 2012 Nov 2. PMID: 23124779; PMCID: PMC3562863.
- Ruiz-Criado, J., Ramos-Barron, M.-A., Fernandez-Fresnedo, G., Rodrigo, E., De Francisco, A.-L. M., Arias, M., & Gomez-Alamillo, C. (2015). Long-Term Mortality among Hospitalized Non-ICU Patients with Acute Kidney Injury Referred to Nephrology. Nephron, 131(1), 23‑33. https://doi.org/10.1159/000437340
- Leaf DE, Christov M. Dysregulated Mineral Metabolism in AKI. Semin Nephrol. 2019 Jan;39(1):41-56. doi: 10.1016/j.semnephrol.2018.10.004. PMID: 30606407.
- Fernandes AR, Viegas MSR, Soares EQ, Coelho SS, Valério P, Barreto JC, Vinhas JM. Outcomes of acute kidney injury in a nephrology ward. Int Urol Nephrol. 2017 Dec;49(12):2185-2193. doi: 10.1007/s11255-017-1716-6. Epub 2017 Oct 11. PMID: 29027072.
- Teo SH, Lee KG, Koniman R, Tng ARK, Liew ZH, Naing TT, Li H, Tan RY, Tan HK, Choong HL, Foo WYM, Kaushik M. A prospective study of clinical characteristics and outcomes of acute kidney injury in a tertiary care Centre. BMC Nephrol. 2019 Jul 26;20(1):282. doi: 10.1186/s12882-019-1466-z. PMID: 31349813; PMCID: PMC6660929.
- Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012 Sep;60(3):402-8. doi: 10.1053/j.ajkd.2012.03.014. Epub 2012 Apr 27. PMID: 22541737; PMCID: PMC3422603.
- Brito GA, Balbi AL, Abrão JM, Ponce D. Long-term outcome of patients followed by nephrologists after an acute tubular necrosis episode. Int J Nephrol. 2012;2012:361528. doi: 10.1155/2012/361528. Epub 2012 Nov 27. PMID: 23227335; PMCID: PMC3514833.
- Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011 Jun;79(12):1361-9. doi: 10.1038/ki.2011.42. Epub 2011 Mar 23. PMID: 21430640; PMCID: PMC3257034.
- Liaño F, Felipe C, Tenorio MT, Rivera M, Abraira V, Sáez-de-Urturi JM, Ocaña J, Fuentes C, Severiano S. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007 Apr;71(7):679-86. doi: 10.1038/sj.ki.5002086. Epub 2007 Jan 31. PMID: 17264879.
- Ponte B, Felipe C, Muriel A, Tenorio MT, Liaño F. Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant. 2008 Dec;23(12):3859-66. doi: 10.1093/ndt/gfn398. Epub 2008 Jul 15. PMID: 18632586.
- Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, Lebert C, Bohé J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network. Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med. 2018 Oct 11;379(15):1431-1442. doi: 10.1056/NEJMoa1803213. PMID: 30304656.