More Information
Submitted: September 19, 2023 | Approved: October 04, 2023 | Published: October 05, 2023
How to cite this article: Morales JC, Sias G, Manzoni M, Loriga G. A Case of Catastrophic Atypical Hemolytic Uremic Syndrome Unresponsive to Eculizumab and the use of Ravulizumab Off-label. J Clini Nephrol. 2023; 7: 073-077.
DOI: 10.29328/journal.jcn.1001113
Copyright License: © 2023 Morales JC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Atypical hemolytic uremic syndrome; Myopericarditis; Eculizumab; Ravulizumab; Plasma exchange; C5 blockade; End-stage chronic kidney disease
A Case of Catastrophic Atypical Hemolytic Uremic Syndrome Unresponsive to Eculizumab and the use of Ravulizumab Off-label
Jorge Cabrera Morales1*, Giuseppe Sias1, Marco Manzoni2 and Giacomina Loriga1
1Department of Nephrology, Santissima Annunziata Hospital, Sassari, Italy
2Department of Cardiology, Santissima Annunziata Hospital, Sassari, Italy
*Address for Correspondence: Jorge Cabrera Morales, Department of Nephrology, Santissima Annunziata Hospital, Sassari, Italy, Email: [email protected]
“A 40-year-old woman with melanoma, under treatment with Dabrafenib and Trametinib, was evaluated in our hospital for rapidly progressive deterioration of renal function”.
8 months before the current admission, the patient had been diagnosed with melanoma, and underwent radical surgery and subsequent therapy with Dabrafenib and Trametinib.
After 5 months of therapy, the patient was brought to this hospital for precordial pain, with a diagnosis of myopericarditis, therapy was started for heart failure with a good response. However, the patient developed a progressive impairment of renal function, associated with hemolytic anemia and thrombocytopenia. The peripheral smear showed the presence of schistocytes.
The suspicion of atypical Hemolytic Uremic Syndrome (aHUS) was confirmed by the assay of C5B-9 induced by serum on endothelial cells, which showed a deposition of 331%, treatment with Eculizumab was initiated.
After 3 administrations the patient did not improve, with further worsening of the hemolytic condition, and progression of renal damage.
Due to the failure of Eculizumab, we considered the use of Ravulizumab. However, in Italy only can be administered to patients in Eculizumab stable treatment for at least three months. Nevertheless, faced with the catastrophic condition, it was decided to shift the therapy and use off-label Ravulizumab. After 10 days of the first administration, the laboratory tests showed a continuous rise in the values of haptoglobin, platelets, and hemoglobin, and a decrease in LDH. The renal function failed to return to normal values but after 20 days of therapy with Ravulizumab, there was complete resolution of the hemolytic condition.
Hemolytic Uremic Syndrome (HUS) is a rare clinical entity distinguished by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and organ damage [1]. HUS is commonly classified into two major subtypes: typical, caused by Shiga toxin-producing Escherichia Coli infection (STEC), predominantly affects children [2], and the atypical form (aHUS), which constitutes approximately 5% - 10% of HUS cases and arises due to dysregulation of the C3 convertase within the alternative complement pathway, and secondary forms of HUS may manifest concomitantly with other underlying conditions, such as autoimmunity, transplantation, malignancy, infections, or the use of specific cytotoxic agents, in which complement is involved in some cases, roughly 10% of afflicted individuals experience complications encompassing myocardial infarction, myocarditis, heart failure, cardiomyopathy, and occlusive coronary artery disease [3].
The blockade of complement component 5 (C5) has emerged as the cornerstone of the treatment of aHUS. In 2011, Eculizumab, a humanized monoclonal anti-C5 antibody, was introduced as a pivotal intervention that effectively inhibits terminal complement pathway activation. Eculizumab has demonstrated notable success in nearly all instances of aHUS and a favorable efficacy profile for many secondary forms [4]. Although the literature contains limited reports of Eculizumab therapy failure in patients with aHUS [5]. In December 2018, Ravulizumab, a long-acting inhibitor of complement component 5 (C5), garnered global approval for the treatment of aHUS, offering improved quality of life [6]. Nevertheless, in Italy, the utilization of Ravulizumab is selectively reserved for patients who have maintained a stable Eculizumab regimen for a minimum of three months due to financial considerations.
We describe a clinical case of a young woman with a diagnosis of melanoma who underwent surgery and chemotherapy, and who developed a catastrophic form of atypical hemolytic uremic syndrome, in which the treatment with Eculizumab was unsuccessful.
A 40-year-old woman with a recent metastatic melanoma, under treatment with Dabrafenib and Trametinib, after radical surgery, was evaluated in our hospital for rapidly progressive deterioration of renal function.
Approximately 8 months before the current admission, the patient had been diagnosed with melanoma. She underwent surgery to resection the skin neoplasm with inguinal lymphadenectomy. Subsequently, she initiated treatment with Dabrafenib and Trametinib.
Three months before the current admission the patient presented an episode of precordial pain and was admitted to the cardiology department with a diagnosis of myocarditis, she stopped the therapy for melanoma and underwent myocardial treatment, after 1 month the patient was discharged from the hospital, and therapy for melanoma was resumed.
For the recurrence of the symptoms, the patient was brought to the cardiology department of this hospital, presenting with recurrent myopericarditis, she was admitted to the cardiology department. Transthoracic echocardiogram revealed a dilated left ventricle with widespread hypokinesia and reduced ejection fraction (EF: 46%); indirect signs of increased filling pressures and enlarged inferior vena cava were also present at the echocardiography. She was treated with ibuprofen and colchicine for the pericarditis and with ACE-I, beta-blockers, and diuretics for the heart failure due to the myocardial involvement with good response to cardiologic function. However, the patient developed a progressive impairment of renal function, associated with hemolytic anemia with consumption thrombocytopenia (Table 1).
| Table 1: The patient provided written consent for the publication of this case. | |||
| Laboratory | Autoimmunity | ||
| Hb: | 8.70 g/dL | AMA | Negative |
| MCV | 68.4 fL | ANA | Positive |
| MCH | 21.3 pg | P-ANCA | Negative |
| Reticulocyte | 6.7% | C-ANCA | Negative |
| Total bilirubin | 1 mg/dc | Anti Jo1 | Negative |
| PLT | 89 x 10/3 uL | RNP | Negative |
| LDH | 483 U/L | Scl 70 | Positive |
| Creatinine | 3.74 mg/dL | Anti-SM | Negative |
| Azotemia | 55 mg/dL | SS A/Ro | Negative |
| Haptoglobin | 59 mg/dL | Anti SS B/La | Negative |
| Coombs | Negative | ADAMST 13 | Normal |
| C3 | 76 mg/dL | ||
| C4 | 8 mg/dL | ||
The peripheral smear showed the presence of schistocytes, consistent with microangiopathy.
At the time of the diagnosis of microangiopathy, the aHUS had not been confirmed. Furthermore, eculizumab was not available in the hospital at that time, so according to the ASFA guidelines, which recommend: “before a diagnosis of aHUS is confirmed, the standard of care is to initiate Therapeutic Plasma Exchange (TPE)” [7]. The patient underwent TPE, which yielded a positive response. Subsequently, she was transferred to the nephrology department.
The suspicion of atypical Hemolytic Uremic Syndrome (aHUS) was confirmed through an assay measuring C5B-9 deposition induced by serum on endothelial cells, which revealed deposition of 331% (normal value < 150%).
Testing for antibodies against factor H by ELISA was performed and yielded negative results. Subsequently, treatment with Eculizumab was initiated.
A renal biopsy was performed on the patient, revealing the following findings: Expansion of the mesangial region with thickening of the basement membranes, displaying a “double contour” profile, indicative of a membranous proliferative pattern, evidence of microthrombosis in the afferent arterioles, presence of fibrin deposition and fibrinoid necrosis and in the interstitial area, a focal lymphocytic infiltrate was observed, with occasional involvement of the epithelium of the tubules.
After 3 administrations of Eculizumab, the plasma exchange was stopped. Unfortunately, the patient’s condition did not improve; instead, there was a further deterioration of hemolytic symptoms and progressive renal damage, necessitating hemodialysis. In an effort to address this critical situation, emergency therapy with plasma exchange was reinstated, albeit with only partial success. However, after the seventh plasma exchange session, the response became notably poor, with the potential for more risks than benefits.
In an effort to address renal inflammation, as indicated by the biopsy findings and potentially linked to chemotherapy drug toxicity, prednisone therapy was initiated at a dosage of 0.5 mg/kg/day. Despite these interventions, renal function failed to improve, and the patient developed malignant hypertension (BP: 180/110 mmHg), which exhibited a very poor response to antihypertensive therapy and exacerbated microangiopathic hemolysis.
Due to the ineffectiveness of Eculizumab therapy and the persistent severe hemolysis that no longer responded to plasma exchange, we decided to shift the treatment and use Ravulizumab, which has demonstrated benefits comparable to Eculizumab [8]. Nevertheless, in Italy, due to financial constraints, the product’s label indicates that it can only be administered to patients who have maintained a stable Eculizumab regimen for at least three months. In light of the patient’s catastrophic condition and the imminent risk of death, the decision was made to proceed with the off-label use of Ravulizumab.
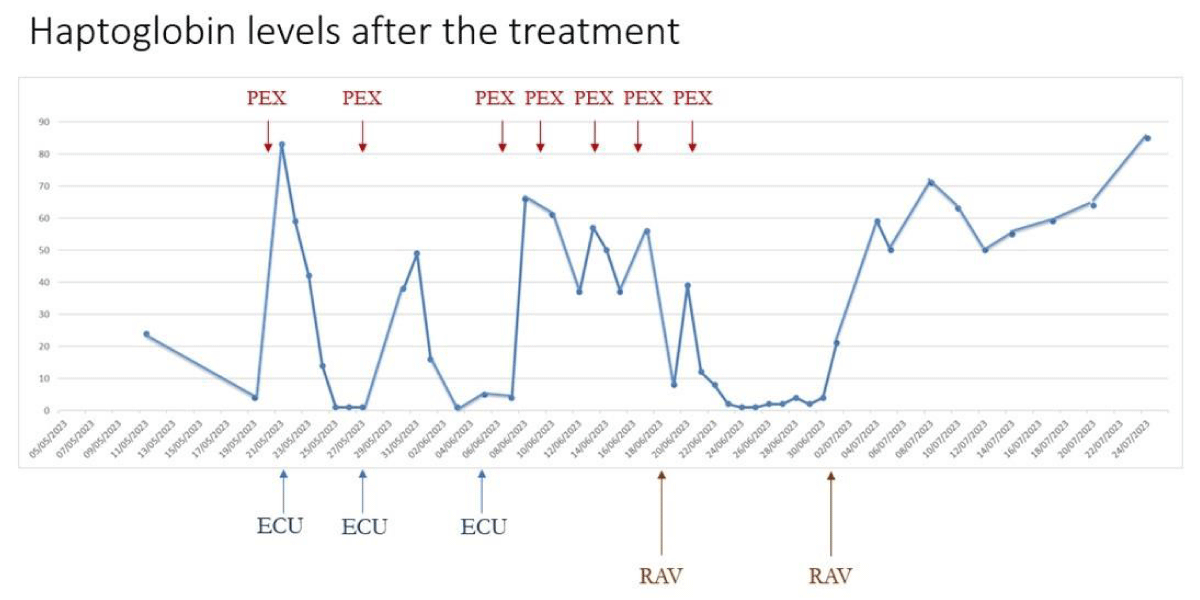
Following the initial administration of Ravulizumab, the patient did not exhibit an immediate response to hemolysis. However, ten days later, a second administration was performed, and within two days of this infusion, laboratory tests indicated a continuous increase in haptoglobin, platelet count, and hemoglobin levels, along with a decrease in lactate dehydrogenase (LDH) levels. After twenty days from the second administration, laboratory tests normalized, and the hemolytic condition was completely resolved. The response can be observed in the increase in haptoglobin levels, as illustrated in Figure 1.
Figure 1: Haptoglobin levels after the treatment. PEX: Plasma Exchange; ECU: Eculizumab; RAV: Ravulizumab.
Renal function did not return to normal levels, resulting in End-Stage Chronic Kidney Disease (ESCKD), which required the initiation of dialysis therapy. The patient underwent dialysis with good tolerance and without complications.
At present, the patient is receiving Ravulizumab, with regular hemolysis assessments well under control, and she continues to tolerate the treatment effectively.
Atypical Hemolytic Uremic Syndrome (aHUS) is a rare multiorgan disorder associated with unfavorable outcomes if left untreated. Over the past decade, there has been significant progress in comprehending and managing aHUS. Consequently, prospective non-randomized studies and retrospective series have provided evidence of the effectiveness of treatments targeting complement component C5, which inhibits the formation of the C5b-induced membrane attack complex. Nevertheless, the exact range of complement-mediated thrombotic microangiopathy remains a topic of ongoing debate.
We are in front of a case of a 44-year-old woman with severe heart and kidney involvement, hemolytic anemia, and consumption of the complement. Consequently, this case can be categorized as secondary atypical Hemolytic Uremic Syndrome (aHUS), in which the triggers can be countless. Upon evaluating the chronological sequence of events, it becomes evident that the clinical manifestations manifested following the administration of chemotherapy for melanoma (Dabrafenib and Trametinib). These therapeutic agents are known to be associated with various side effects, with cardiotoxicity being one of the most extensively studied in this context [9-11].
While cases of drug-induced Thrombotic Microangio-pathy (TMA) secondary to tyrosine kinase inhibitor chemotherapy have been reported [12]. it is worth noting that microangiopathy could potentially be responsible for the cardiac damage observed in this patient. Individuals with mutations in CFH and C3 genes are known to have an elevated risk of coronary vasculature involvement [3]. It is possible that heart damage preceded kidney involvement. Consequently, during the patient’s first cardiac event, suspicion of microangiopathy was not raised, due to the absence of striking hemolytic manifestations, which only emerged later. Although the association between Dabrafenib/Trametinib and the development of TMA is still under investigation [6]. It can reasonably be inferred as a potential contributing factor in this case.
The delay in recognition of atypical hemolytic uremic syndrome (aUSS) may be attributed to the simultaneous appearance of several clinical factors. Notably, the patient had concomitant heart failure, possibly secondary to the administration of Dabrafenib and Trametinib, which led to an initial focus on addressing hypoperfusion and evaluating potential drug-related toxicity. Consequently, primary diagnostic consideration initially overlooked SEU as a possible etiology of the observed acute kidney injury.
At the time of the microangiopathy diagnosis, Eculizumab was unavailable in the hospital. Following ASFA guidelines, Therapeutic Plasma Exchange (TPE) was initiated as an alternative treatment. However, when Eculizumab failed to yield the expected results, we made the decision to urgently resume TPE, which led to a partial response.
The non-response to Eculizumab in the context of full-blown atypical Hemolytic Uremic Syndrome (aHUS) is an uncommon and unexpected occurrence. In a database study covering the period from 2007 to 2019, involving 69 cases of drug-induced microangiopathy treated with Eculizumab, it was found that 80% achieved renal recovery, 59% were able to discontinue hemodialysis, and 74% attained complete hematologic recovery [14]. Nonetheless, there have been a few reported instances of poor response to Eculizumab in cases of microangiopathies [5].
Our patient exhibited persistent hemolysis following Eculizumab treatment, resulting in a catastrophic scenario with an imminent risk of death. Given that there is another monoclonal antibody, Ravulizumab, indicated for aHUS and demonstrating favorable responses [15]. we opted to transition to this treatment. Remarkably, after just 11 days of initiating Ravulizumab therapy, laboratory tests indicated a gradual resolution of hemolysis, ultimately achieving complete resolution, thus demonstrating the success of Ravulizumab treatment.
One hypothesis for this success is the differential blocking action of Eculizumab and Ravulizumab on factor C5. Eculizumab has been observed to incompletely block factor C5, while Ravulizumab achieves complete inhibition [16]. We initially considered the presence of a positive Scl70 and complement consumption as potential manifestations of Systemic Lupus Erythematosus (SLE). However, given the absence of other diagnostic criteria and the evident positive response to Ravulizumab, we ruled out the SLE diagnosis [17].
Regrettably, the kidney damage did not regress, necessitating the initiation of dialysis therapy. Nonetheless, this intervention ultimately saved the patient’s life.
While we cannot definitively identify the precise trigger for the onset of atypical Hemolytic Uremic Syndrome (aHUS), we present a noteworthy case of thrombotic microangiopathy that occurred following the administration of melanoma chemotherapy (Dabrafenib/Trametinib). Further investigations are warranted to validate this potential association.
This case represents a distinct and severe manifestation of aHUS, wherein Ravulizumab has demonstrated superior effectiveness in halting hemolysis when compared to the complete ineffectiveness of Eculizumab.
None of the authors have any professional relationships with companies or manufacturers that would benefit from the results of the present study.
The authors wish to express their gratitude to all the staff of the Nephrology Division at Santissima Annunziata Hospital, Sassari, Italy.
- Mazzierli T, Allegretta F, Maffini E, Allinovi M. Drug-induced thrombotic microangiopathy: An updated review of causative drugs, pathophysiology, and management. Front Pharmacol. 2023 Jan 9;13:1088031. doi: 10.3389/fphar.2022.1088031. PMID: 36699080; PMCID: PMC9868185.
- Loirat C, Frémeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet J Rare Dis. 2011 Sep 8;6:60. doi: 10.1186/1750-1172-6-60. PMID: 21902819; PMCID: PMC3198674.
- Raina R, Krishnappa V, Blaha T, Kann T, Hein W, Burke L, Bagga A. Atypical Hemolytic-Uremic Syndrome: An Update on Pathophysiology, Diagnosis, and Treatment. Ther Apher Dial. 2019 Feb;23(1):4-21. doi: 10.1111/1744-9987.12763. Epub 2018 Oct 29. PMID: 30294946.
- Tschumi S, Gugger M, Bucher BS, Riedl M, Simonetti GD. Eculizumab in atypical hemolytic uremic syndrome: long-term clinical course and histological findings. Pediatr Nephrol. 2011 Nov;26(11):2085-8. doi: 10.1007/s00467-011-1989-4. Epub 2011 Aug 30. PMID: 21877169.
- Nishimura J, Yamamoto M, Hayashi S, Ohyashiki K, Ando K, Brodsky AL, Noji H, Kitamura K, Eto T, Takahashi T, Masuko M, Matsumoto T, Wano Y, Shichishima T, Shibayama H, Hase M, Li L, Johnson K, Lazarowski A, Tamburini P, Inazawa J, Kinoshita T, Kanakura Y. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014 Feb 13;370(7):632-9. doi: 10.1056/NEJMoa1311084. PMID: 24521109.
- McKeage K. Ravulizumab: First Global Approval. Drugs. 2019 Feb;79(3):347-352. doi: 10.1007/s40265-019-01068-2. PMID: 30767127.
- Connelly-Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, Patriquin CJ, Pham HP, Sanchez AP, Schneiderman J, Witt V, Zantek ND, Dunbar NM. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice - Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Ninth Special Issue. J Clin Apher. 2023 Apr;38(2):77-278. doi: 10.1002/jca.22043. PMID: 37017433.
- Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, Pessoa V, Gualandro S, Füreder W, Ptushkin V, Rottinghaus ST, Volles L, Shafner L, Aguzzi R, Pradhan R, Schrezenmeier H, Hill A. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019 Feb 7;133(6):530-539. doi: 10.1182/blood-2018-09-876136. Epub 2018 Dec 3. PMID: 30510080; PMCID: PMC6367644.
- Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol. 2017 Oct;17(4):487-493. doi: 10.1007/s12012-017-9425-z. PMID: 28861837; PMCID: PMC6319910.
- Banks M, Crowell K, Proctor A, Jensen BC. Cardiovascular Effects of the MEK Inhibitor, Trametinib: A Case Report, Literature Review, and Consideration of Mechanism. Cardiovasc Toxicol. 2017 Oct;17(4):487-493. doi: 10.1007/s12012-017-9425-z. PMID: 28861837; PMCID: PMC6319910.
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022 Nov 1;43(41):4229-4361. doi: 10.1093/eurheartj/ehac244. Erratum in: Eur Heart J. 2023 May 7;44(18):1621. PMID: 36017568.
- Reese JA, Bougie DW, Curtis BR, Terrell DR, Vesely SK, Aster RH, George JN. Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015 May;90(5):406-10. doi: 10.1002/ajh.23960. Epub 2015 Feb 25. PMID: 25639727; PMCID: PMC4409501.
- Akino S, Ohashi H, Okano T, Takeuchi S, Kawakami T, Soma Y, Kadono T. Sudden elevation of plasma D-dimer levels induced by the combination therapy of dabrafenib and trametinib: Report of two cases. J Dermatol. 2019 Apr;46(4):358-360. doi: 10.1111/1346-8138.14798. Epub 2019 Feb 5. PMID: 30719722.
- Zafar A, Lim MY, Abou-Ismail MY. Eculizumab in the management of drug-induced thrombotic microangiopathy: A scoping review of the literature. Thromb Res. 2023 Apr;224:73-79. doi: 10.1016/j.thromres.2023.02.012. Epub 2023 Mar 2. PMID: 36871347.
- Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, Miyakawa Y, Ortiz S, Swenson E, Vallee M, Yoon SS, Kavanagh D, Haller H; 311 Study Group. The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020 Jun;97(6):1287-1296. doi: 10.1016/j.kint.2020.01.035. Epub 2020 Mar 6. Erratum in: Kidney Int. 2020 Dec;98(6):1621. Erratum in: Kidney Int. 2021 May;99(5):1244. PMID: 32299680.
- Peffault de Latour R, Brodsky RA, Ortiz S, Risitano AM, Jang JH, Hillmen P, Kulagin AD, Kulasekararaj AG, Rottinghaus ST, Aguzzi R, Gao X, Wells RA, Szer J. Pharmacokinetic and pharmacodynamic effects of ravulizumab and eculizumab on complement component 5 in adults with paroxysmal nocturnal haemoglobinuria: results of two phase 3 randomised, multicentre studies. Br J Haematol. 2020 Nov;191(3):476-485. doi: 10.1111/bjh.16711. Epub 2020 May 24. PMID: 32449174; PMCID: PMC7687070.
- Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019 Sep;71(9):1400-1412. doi: 10.1002/art.40930. Epub 2019 Aug 6. PMID: 31385462; PMCID: PMC6827566.