More Information
Submitted: December 27, 2021 | Approved: April 24, 2023 | Published: April 25, 2023
How to cite this article: Manuti JK, Saadoon AM, Jawad TS, Alawn AG. Evaluation of catheter related bacteremia in patients with end stage renal disease on hemodialysis. J Clini Nephrol. 2023; 7: 032-041.
DOI: 10.29328/journal.jcn.1001105
Copyright License: © 2023 Manuti JK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Catheter; Hemodialysis; Bacteremia
Evaluation of catheter related bacteremia in patients with end stage renal disease on hemodialysis
Jawad K Manuti*, Ali Mohammed Saadoon, Talal Shakir Jawad and Ali Ghafil Alawn
Al-Nahrain University, Iraq
*Address for Correspondence: Dr. Jawad K Manuti, F.I.C.M.S, Professor of Medicine, Dialysis Unit, Department of Medicine, AL-Nahrain University-Medical College Institution, Baghdad, Iraq, Email: [email protected]
Infections are common complications among patients on chronic hemodialysis. Hemodialysis patients with a catheter have a 2- to 3-fold increased risk of hospitalization for infection and death compared with patients with an arteriovenous fistula or graft [1].
Infection is second only to cardiovascular disease as a cause of death in end-stage renal disease. Septicemia accounts for more than 75% of infection-associated death. The arteriovenous fistula is the preferred vascular access for HD because of reduced infections rate and improved delivery of adequate dialysis, unlike cardiovascular catheter (CVC) that has lower patency rate, high infection rate, hospitalization and mortality mainly due to catheter related blood stream infection [2].
The predominant reasons for use of CVCs include: temporary loss of permanent hemodialysis access, late referral for initiation of dialysis, the need to await maturation of Arteriovenous Fistulas (AVFs) and limited access options in patients with severe peripheral vascular disease. On - tunneled CVCs for HD primarily placed in internal jugular, sub-clavian or femoral vein, they are indicated for short-term HD access and their use should be limited to less than three weeks; otherwise if we need it for more than three weeks, a tunneled catheter is the option [3,4].
Types of CVC used for chronic hemodialysis include tunneled cuffed catheters and nontunneled catheters. The risk of developing bacteremia varies with site of CVC insertion, type of device and duration of CVC use. The most common causative pathogens are gram positive bacteria, with Staphylococcus aureus and coagulase- negative staphylococci accounting for 40% to 80% of catheter related blood stream infection (CRBSIs)13) Gram-negative organisms cause 20% to 40% CRBSIs, whereas polymicrobial infections (10% - 20%) and fungal infections (<5%) are less common. Metastatic infectious complications of CRBSIs include endocarditis, osteomyelitis, spinal epidural abscess, septic arthritis, brain abscess, and septic pulmonary emboli [5-13].
Risk Factors for the Occurrence of CRBSIs include: Submaximal barrier precautions at the time of catheter insertion, Nontunneled catheter, Site of insertion—femoral > internal jugular > subclavian, Prolonged duration of catheter use, Previous episode of CRBSI, Staphylococcus aureus nasal carriage, Diabetes, Hypoalbuminemia and Recent surgery.
Clinical Features; Fever and chills are the most sensitive clinical features, associated with positive blood cultures in 60% to 80% of patients [14,15]. Only 5% of patients with CRBSIs will have a concurrent exit-site or tunnel infection [16]. Other clinical manifestations of CRBSIs include hemodynamic instability, altered mental status, catheter dysfunction, hypothermia, nausea/vomiting and generalized malaise [17].
Clinical definitions of CRBSIs are those where other sources of infection are excluded by patient examination and review of patient record and finding of positive catheter tip cultures (if available) with the same organism as that seen on blood cultures [18,19].
Diagnosis without catheter withdrawal; Paired quantitative blood cultures, both sets are positive for the same microorganism and the set obtained through the catheter has ≥3:1 fold-higher colony count than the peripheral culture [18-20] Diagnosis with catheter withdrawal.
Semiquantitative catheter culture ≥15 CFU The same microorganism in at least one percutaneous blood culture and catheter tip culture [18-20].
Catheter cultures should be performed when a catheter is removed for suspected CRBSI; catheter cultures should not be obtained routinely. For Central Venous Catheters (CVCs), the catheter tip should be cultured, rather than the subcutaneous segment [21-27].
Exit-site infection diagnosed by Hyperemia, induration, and/or tenderness ≤2 cm from catheter exit site. May be associated with fever and purulent drainage from the exit site. It may or may not be associated with bacteremia. If there is purulent drainage, it should be collected and sent for Gram staining and culture. Treatment for 7 to 14 days, depending on the microorganism isolated and local practice [17,20].
Tunnel infection diagnosed by Tenderness, hyperemia, and/or induration that extends >2 cm from the exit site and along the subcutaneous tunnel. It may or may not be associated with bacteremia. If there is purulent drainage, it should be collected and sent for Gram staining and culture [20]. The catheter should always be removed, without exchange over a wire. A new catheter should be inserted at a separate site. Start empiric broad-spectrum antibiotics to cover both gram-positive and gram negative organisms.
Modify antibiotic regimen when culture and sensitivity results are available.
Tunnel infections, in the absence of a concurrent CRBSI, are typically treated for 10 to 14 days, depending on the microorganism isolated and local practice. If a CRBSI is also present, then duration of therapy will be determined by the management of the CRBSI [17].
Catheter-Related Bloodstream Infection Empiric management; Broad-spectrum antibiotics should be initiated to cover both gram-positive and gram-negative organisms. Antibiotics should generally cover methicillin resistant S aureus (MRSA) and Pseudomonas. Following initiation of empiric antibiotic therapy, it is crucial that culture and sensitivity data are followed up in a timely manner, so that the most appropriate antibiotics based on sensitivity results can be used [20].
Definitive management of CRBSIs must be tailored to the clinical presentation of the patient, the microorganism isolated, and vascular access options of the patient. For example, management of the patient with septic shock secondary to MRSA CRBSI will differ from that of hemodynamically stable patient presenting with a fever and found to have coagulase-negative staphylococcus. Treatment can be categorized into 3 groups: systemic antibiotics, antimicrobial locking (instillation) solutions and catheter management [17].
Systemic antibiotics; all patients with a CRBSI should receive systemic antibiotics, which will typically be administered for 2 to 6 weeks depending on the microorganism, clinical presentation and complications. Final decision on specific antibiotic agent(s) is dependent on final blood culture result and sensitivities and whether or not patient has any allergies [28-35].
If Methicillin Sensitive S Aureus (MSSA) infection is isolated, cefazolin is the preferred choice over vancomycin because it is associated with decreased hospitalization and death secondary to infection [21].
Study settings and design
A cross - sectional observational study, enrolling (300) ESRD patients in whom tunneled and non-tunneled catheters used for hemodialysis in the Dialysis Center of Al Imamain Alkadhumain Medical City from February 2020 to February 2021 under supervision of Nephrologist. (on regular hemodialysis in this center but not of all them complaining bacteremia) [36-43].
Patients assessments
All 300 patients on regular hemodialysis with three or two sessions per week and 4 hours duration for each session using GAMBRO Dialysis system were evaluated in this study.
Patients selection
Initially, three hundred patients were evaluated in this study, but only 122 of them gave signs and symptoms of catheter related infection, all of them more than 18 years old, using tunneled and non tunneld catheter.
178 patients were excluded from this study, including;
68 patients were asymptomatic.
60 patients were having arteriovenous fistula.
14 patients were receiving antibiotics at the time of blood culture or catheter removal.
30 patients with infection other than catheter related infection such as pneumonia and UTI.
4 patients on immunosuppressive treatment or steroids.
2 patients with malignancy.
Baseline assessment
Including history, physical examination and investigations were done for studied patients according to the following: - age: younger age < 45 years, middle aged group 45 - 65 years and elderly > 65 years old [43], sex, causes of CKD, types of double lumen whether tunneled or non tunneld.
- Site of double lumen (internal jugular, femoral, subclavian), duration of hemodialysis and duration of double lumen (less than 4 weeks and more than 4 weeks), presentation: fever, chills, rigor, nausea and vomiting, unexplained hypotension, change in mental status, local signs of exit site infection and catheter dysfunction, duration of symptoms equal or >3 days or <3 days during HD sessions or during HD free period [44,45].
Investigations: WBC (neutrophil), HB, B.urea, s.cr, RBS, s.albumin, blood culture, catheter tip culture, CxR, GUE .
- Hb level: ˂ 8 g/dL (sever), 8 – 10.9 g/dl (moderate) and ≥ 11 g/dL(mild) [46].
- Wbc count: 4 – 11 × 109/L (normal), ˃ 11 × 109/L (leukocytosis) [47].
- Neutrophil count (2 - 8× 109/L (normal), ˃ 8 × 109/dL (neutrophilia) [47].
- Albumin level: normal s. albumin ≥ 3.5 g/dL, hypoalbuminemia < 3.5 g/dl [45].
- Random blood glucose : 11.1 mmol/L (200 mg/dl) or more
- {Hyperglycemia}, <11.1 mmol/L (200mg/dl) {normal} [44]. (all these investigations routinely done for all the patients in dialysis to assessment the health of patients)
- Hx of previously receiving antibiotics and for how long duration, Treatment whether need antibiotics only (systemic antibiotics and antibiotics lock) or removal of catheter plus antibiotics.
- We remove the double lumen and cut nearly 4 cm from the tip and place it in sterile container, then transported to the lab for culture [48].
- We use catheter hub and at least one peripheral venous site for (blood culture and sensitivity).
- Blood culture and sensitivity were done in the device by Minimum Inhibitory Concentration (MIC).
Data collection
A preformed Questionnaire was used to get information from studied population. Blood samples were taken at dialysis units and investigated in Alkadhumain medical city / laboratory department.
Ethical issues
The patients will be informed about the study purpose and its relevance and their verbal consent had been taken to conduct the study. Ethical Approved by Iraq board internal medicine committee.
Statistical analysis
The data analyzed using Statistical Package for Social Sciences (SPSS) version 22. The data presented as mean, standard deviation and ranges. Categorical data presented by frequencies and percentages. Independent ttest (two tailed) was used to compare the continuous variables among study groups accordingly. Pearson’s Chi–square test was used to assess statistical association between categorical variables. Multivariate regression analysis was conducted to identify the significant unconfounded factors associated with the controlled status of DM. A level of p – value < 0.05 was considered significant.
There 300 patients included in this study. The mean age was 56.7 ± 13 years (range 23 – 80). Males and female were represented 49.3% (148), 50.7% (152) respectively. The features shown in Tables 1,2.
| Table 1: Demographic features of patients (No. 300). | ||
| Variable | Frequency Percentage | |
| Age | Mean ± SD - 56.7 ± 13 Years | |
| Duration of Dialysis | Mean ± SD - 3.2 ± 2 Years | |
| Sex Male Female |
148 152 |
49.3% 50.7% |
| Type of vascular access Double lumen Arteriovenous fistula |
240 60 |
80.0 20.0 |
| Site of lumen Femoral vein Internal Jugular vein Subclavian vein |
33 202 5 |
13.7 84.1 2.9 |
| Type of Double lumen Non Tunneled Tunneled |
105 195 |
35.0 65.0 |
| Duration of Double Lumen < 4 weeks > 4 weeks |
163 137 |
54.3 45.7 |
| Causes of chronic kidney disease Diabetic Nephropathy Hypertention/Diabetis mellitus Hypertention Systemic lupus erythmatosus Polycystic kidney disease Unknown cause |
125 54 50 5 12 54 |
41.7 18.0 16.7 1.7 4.0 18.0 |
| Table 2: Clinical manifestation among patients. | |||
| Symptoms | During HD (n = 122) N. (%) |
Free of HD (n = 82) | |
| N. (%) | p value* | ||
| Fever with/without chills Yes |
122 (100) |
82 (100) |
-- |
| Nausea and vomiting Yes No |
31 (25.4) 91 (74.6) |
21 (25.6) 61 (74.4) |
0.94 |
| Unexplained hypotension Yes No |
8 (6.6) 114 (93.4) |
8 (9.8) 74 (90.2) |
0.041 |
| Change in mental state Yes No |
6 (4.9) 116 (95.1) |
6 (7.3) 76 (92.7) |
0.79 |
| Catheter dysfunction Yes No |
10 (8.2) 112 (91.8) |
10 (12.2) 72 (87.8) |
0.021 |
| Local signs of exit site or tunnel infection Yes No |
24 (19.7) 98 (80.3) |
14 (17.2) 68 (82.9) |
0.31 |
| *Chi-Square test (within Free of HD group only). | |||
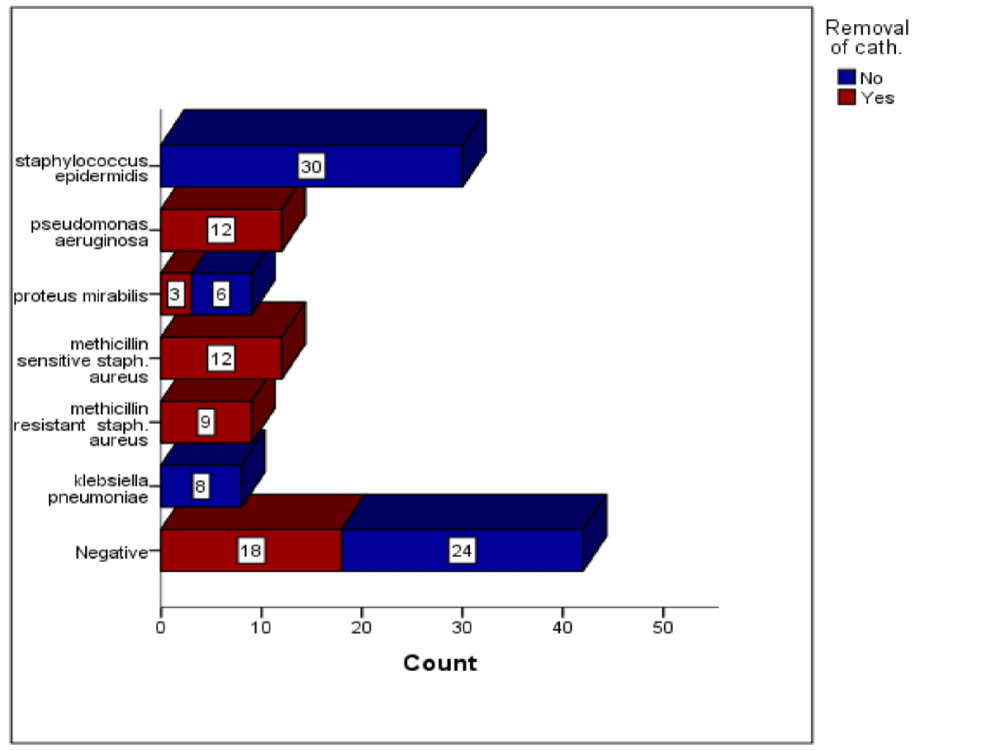
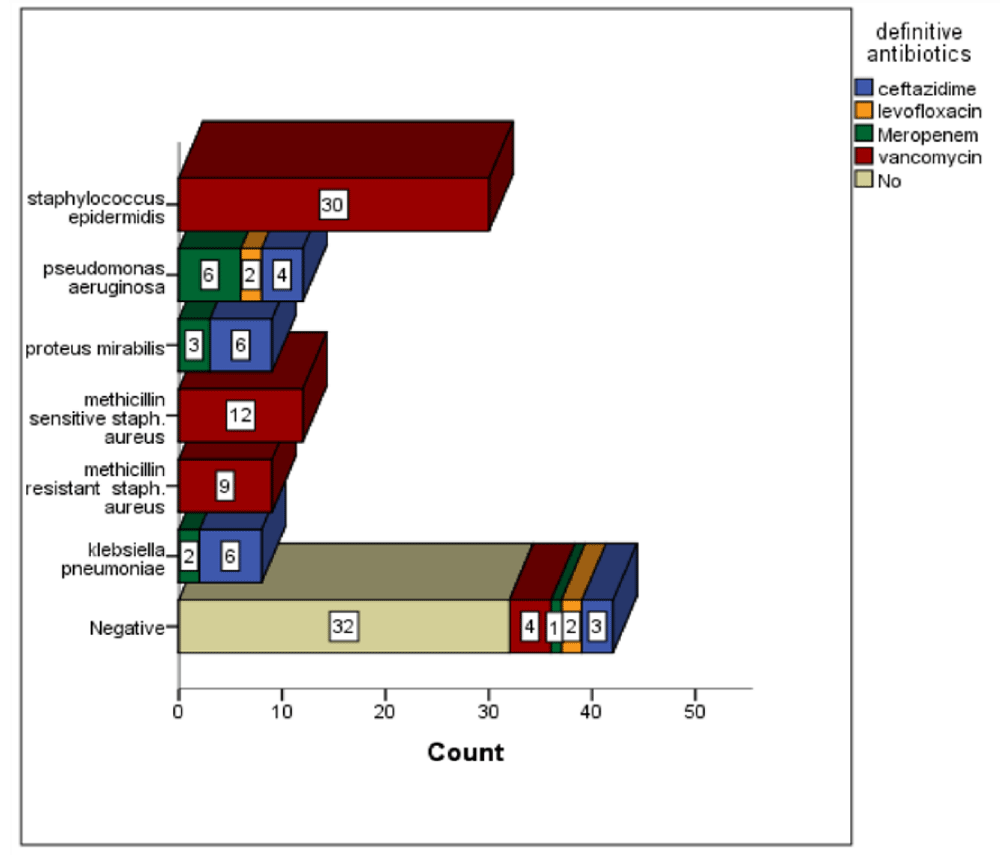
The most common bacteria identified in blood culture was staphylococcus epidermidis, which was presented in 30 (24.5%) patients. Then pseudomonas aeruginosa and methicillin sensitive staph. aureus which were presented in 12 (9.8%) patients for each. Then methicillin resistance staph. aureus and proteus mirabilis, which were found in 9 (7.3%) patients for each bacteria [49-55]. Klebsiella pneumonia was presented in 8 (6.2%) patients. There were 42 (34.4%) patients have negative results on blood culture Figures 1-3.
Figure 1: The Distribution of catheter removal across the type of bacteria identified in the blood culture. Chi-Squ = 72.2, p value = 0.0001. Chi-Squ = 113.3, p value = 0.0001
Figure 2: The Distribution of definitive antibiotics across the type of bacteria identified in the blood culture. Chi-Squ = 199.1, p value = 0.0001.
Figure 1: The Distribution of definitive antibiotics across the type of bacteria identified in the catheter tip culture. Chi-Squ = 133.5, p value = 0.0001.
Positive blood culture was associated with lower duration of symptoms (p value = 0.043), with higher WBC and neutrophilia (p value < 0.0001), and with lower S.creatinine and s.albumin (p value = 0.01 and 0.008 respectively). The age, Hb, B.urea, and RBS did not showed an association with positive blood culture [56-58].
The analysis of association between the blood culture and causes of CKD shown no association between the causes of CKD and positivity of blood culture, p value = 0.58.
The catheter tip culture was done for 58 (47.5%) patients, it revealed that pseudomonas aeruginosa was the most common bacteria, identified in 14 (11.4%) patients. Methicillin sensitive staph. aureus was presented in 12 (9.8%) patients. MRSA was presented in 9 (7.3%) patients. Negative results were observed in 12 (9.8%) patients.
The assessment of the factors associated with positive catheter tip culture revealed that, positive catheter tip culture was associated with higher WBC, neutrophilia, and RBS (p value < 0.0001). The age, duration of symptoms, Hb, B.urea, s.creatinine and s.albumin did not showed an association with positive catheter tip culture.
The association between patients with blood culture and catheter tip culture bacteria are shown in Table 3. There was a strong association between the blood culture and catheter tip culture bacteria (p value = 0.001).
| Table 3: The distribution of patients according to blood culture and catheter tip culture bacteria. | |||||||||
| Blood Culture | Catheter Tip Culture | ||||||||
| klebsiella | methicillin resistant |
methicillin sensitive |
-ve | Not done | proteus mirabilis | aeruginosa | epidermidis | Total | |
| klebsiella pneumoniae | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 8 |
| Methicillin resistant | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Methicillin sensitive | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 12 |
| Negative | 3 | 0 | 0 | 8 | 24 | 1 | 2 | 4 | 42 |
| proteus mirabilis | 0 | 0 | 0 | 0 | 6 | 3 | 0 | 0 | 9 |
| pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 12 |
| staphylococcus epidermidis | 0 | 0 | 0 | 4 | 26 | 0 | 0 | 0 | 30 |
| Total | 3 | 9 | 12 | 12 | 64 | 4 | 14 | 4 | 122 |
| Chi-Square= 391.8, p value = 0.001. | |||||||||
All patients had received anempirical systemic antibiotics (vancomycin + ceftazidime). The definitive antibiotics and duration of antibiotics was given in the Table 4.
| Table 4. Duration of systemic antibiotics. | ||
| Variable | Incidence | Percentage |
|
19 4 55 12 32 |
15.6 3.3 45.1 9.8 26.2 |
| Duration 2 weeks 4 weeks 4 – 6 weeks No |
64 12 14 32 |
52.5 9.8 11.5 26.2 |
| Removal of Catheter Yes No |
54 68 |
44.3 55.7 |
| Antibiotics lock Yes No |
44 78 |
36.1 63.9 |
Treatment association with blood culture was given in the below Table 5. There were a strong association between positive culture and definitive antibiotic, duration of antibiotic and antibiotic lock (p value < 0.005).
| Table 5: Association between blood culture and type of treatment. | |||
| Blood culture | |||
| Variable | Positive No. (%) | Negative No. (%) | p value* |
| Removal of Cath. | 36 (66.7) | 18 (33.3%) | 0.82 |
| Definitive Antibiotics Ceftazidime Levofloxacin Vancomycin Meropenem No |
16 (84.2) 2 (50) 51 (92.7) 11 (91.7) 0 |
3 (15.8) 2 (50) 4 (7.3) 1 (8.3) 32 (100) |
0.0001 |
| D 2 weeks 4 weeks 4 – 6 weeks No |
54 (84.4) 14 (100) 12 (100) 0 |
10 (15.6) 0 0 32 (100) |
0.0001 |
| Antibiotics lock Yes No |
44 (100) 36 (46.2) |
0 4242 (53.8) |
0.0001 |
| *Chi-Square Test. | |||
Patients with CKD on hemodialysis are at increased risk for blood stream infection especially those with catheters. In this research, we aimed to investigate the frequency and factors associated with catheter-related bacteremia in patients with CKD on hemodialysis.
The age and sex were comparable to previously published local study by Jaudah and Musa from AlBasra, in which they reported a mean age of 54 years and 55% of their patients were females [56]. The global study from USA at 2019 showed a relatively lower mean age (50 years) in comparison to our study, however, the difference in sample size could contributed to this difference [57].
The most common cause for CKD was diabetic nephropathy followed by hypertension, while the unknown cause was reported in less than 20% of patients, this was comparable to a previous study conducted in Iraq by Awad, in which he studied the causes of CKD [58]. However, in a study conducted among the Iranian population in 2009, they reported that hypertension was relatively higher than DM as a cause for CKD (30.5% and 30.1% respectively) [59]. This difference could rely on the number of patients recruited in their study which was double of our patients, also, ethnicity could play a role too.
Around 40% of recruited patients develop signs/symptoms of infection.
This percentage was comparable to Sanavi’s, et al. study, which showed a 41% of their patients developed an infection [60]. Another Samani’s, et al. study showed that less than 20% of patients developed an infection [61].
While studies from the USA and Italy reported a higher incidence rate of a catheter-related infection (more than 70%) in comparison to our results [57,62]. This differences in studies could be related to difference in the studies design and the precautions measures taken by different institutes to decrease the infection rate.
The presenting symptoms in the majority of patients were fever and rigors in which the bacterial infections are presented with a fever the most, another study by Farrington CA and Allon M to assess the Complications of hemodialysis catheter blood stream infections: impact of infecting organism also reported that more than 90% of their patients presented with fever and rigors [57]. This gave us the assurance of presenting symptoms during HD or not related to HD should take into consideration for identifying the potential catheter-related infection in HD patients.
Among patients who developed sings/symptoms of infection (122), the blood culture showed that 65% of patients have positive culture. The most common bacteria identified in blood culture was staphylococcus epidermidis, while the staph aureus was reported in 16% of patients. This was comparable to a local study by Jaudah and Musa from AlBasra, which showed the most common blood culture organism was staphylococcus epidermidis [56]. In USA, Farrington CA and Allon M found that the staphylococcus epidermidis was the most common organism identified also [57]. Also, Hadian’s et study showed that staphylococcus epidermidis was most common followed by staph aureus [63].
While in study conducted in Canada by Lok CE and Mokrzycki MH, it showed that the staph aureus is the most common identified bacteria across Canada [64].
Also, Sanavi’s, et al. from Iran reported that 42% of isolated organisms was staph aureus [5]. Samani’s, et al. study also showed that staph aureus was the most identified bacteria by blood culture [62].
In narrative review study in Canada by Lata C, et al. to assess the Catheterrelated bloodstream infection in end-stage kidney disease found that the staph aureus bacteria was reported in 31% of patients in which it was the most common bacteria [65].
The short duration of symptoms, leukocytosis, neutrophilia, lowers creatinine, and albumin have been associated with a positive catheter related blood stream infections incidence rate. The binary logistic analysis revealed that hypotension, catheter dysfunction and local signs of exit site infection have been associated positive blood culture. Jaudah and Musa from AlBasra, showed association with local sign, also the showed an association with males, DM, central venous catheter duration and fever [56]. In USA study, it reported that the fever was significantly associated with staph aureus in comparison to staphylococcus epidermidis [2]. While Italian study showed that age, gender and type of catheter were associated with increased incidence of blood culture infection [63]. In study conducted in Canada, it showed different factors than factors in our study, one of the factors was hypertension [66].
This discrepancy in results between studies for factors associated with catheter-related infection in HD patients showed us the importance of all factors and all potentially could have effect on patients [67].
The catheter tip culture was done for half of patients only, among them, the positive catheter tip culture was observed in 74% of patient, which was higher in comparison to local study by Jaudah and Musa, in which they reported that 51% of patients have positive catheter tip culture [1], and to a study conducted in Pakistan by Mahmood SN, et al. to assess the Frequency and microbiological profile of catheter-related infections in hemodialysis patients receiving gentamicin as antimicrobial lock therapy for prophylaxis which showed only 33% of patients were have positive catheter tip culture [68].
The catheter tip culture showed that the pseudomonas aeruginosa was the most common bacteria identifies. The local study by Jaudah and Musa from AlBasra, which showed the most common blood culture organism was staphylococcus epidermidis [56].
Binary logistic analysis revealed that gender, duration of dialysis, hypotension, mental involvement, local signs of exit site infection and symptoms during HD free period were have been associated with positive catheter tip culture.
Diabetes acts as an important factor for catheter tip infection, with an increased likelihood of catheter colonization as in Sahli, et al. in 2016 [69]. This result looks accepted by knowing that Diabetes Mellitus will increase the tissue susceptibility to infection [70].
Ghonemy, et al. in 2015 had found a significant relation between hypoalbuminemia and the risk of CTI that was not evident in our study as they take a larger cohort with different comorbidities [71].
In our study, the positive catheter tip culture was associated with leukocytosis as it associated with response to bacterial infection. Jaudah and Musa from AlBasra, did not showed association between the leukocyte count and positive catheter tip culture [56].
The Staphylococcus Aureus (MSSA) and Staphylococcus epidermidis shared approximately the same antibiotic sensitivity for vancomycin and being MDR, comparable to the studies of Katneni and Hedayati [72], Leone and Suter [73], and Sahli, et al. [68]. Unlike the Pseudomonas aeruginosa, Klebsiella and Merabilis that have a different antibiotic sensitivity like that of Gupta, et al. [74].
Regarding the treatment, given the necessity of proper management, we empirically initiate antibiotic therapy as soon as possible, until receiving definite culture results, in hemodialysis patients suspected of bacteremia [63]. In our study, both gram-positive and gram-negative organisms were common. Hence, when initial empirical treatment is indicated, the coverage of both grams positive and gram negative organisms must be considered [75]. The catheter was removed in 44% of patients as a therapeutic intervention, however, with successful antibiotics, the catheter can be preserved [76]. A longer course of tailored antibiotic therapy with catheter removal or exchange is more appropriate in patients with complicated catheterrelated bloodstream infections [67]. So patients without complications, we can preserved the catheter.
Antibiotic Lock Therapy (ALT), in conjunction with systemic antibiotics, is recommended by scientific societies as a treatment of uncomplicated catheterrelated bloodstream infections in hemodynamically stable hemodialysis patients for whom catheter salvage is the goal.
The rationale for this strategy is the eradication of intraluminal biofilms by the highly concentrated antibiotic used in the lock. In this study, we used the antibiotic lock for 36% of patients.
However, the available evidence supporting this recommendation is scanty and only includes small, shortterm, observational studies (most of them singlearm), with different definitions of CRBSI cure and variable followup periods. In this editorial we provide a critical view on the available evidence regarding the efficacy of ALT on the treatment of CRBSI in hemodialysis patients, as well as the microbiological issues and technical challenges of this strategy [77,78].
1. Nearly less than half of hemodialysis patients develop signs/symptoms of infection.
2. The blood culture showed nearly 2 third of patients have positive culture.
3. The most common bacteria identified in blood culture was staphylococcus epidermidis, while the staph aureus was reported in 16% of patients.
4. Positive catheter tip culture was observed in 2 third of the patients.
5. The catheter tip culture showed that the pseudomonas aeruginosa was the most common bacteria identified.
6. Methicillin Sensitive Staph (MSSA) and Staphylococcus epidermidis shared approximately the same antibiotic sensitivity for vancomycin.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001 Oct;60(4):1443-51. doi: 10.1046/j.1523-1755.2001.00947.x. PMID: 11576358.
- Vassalotti JA, Jennings WC, Beathard GA, Neumann M, Caponi S, Fox CH, Spergel LM; Fistula First Breakthrough Initiative Community Education Committee. Fistula first breakthrough initiative: targeting catheter last in fistula first. Semin Dial. 2012 May;25(3):303-10. doi: 10.1111/j.1525-139X.2012.01069.x. Epub 2012 Apr 4. PMID: 22487024.
- Allon M, Work J. Venous Catheter Access for Hemodialysis. In: Daugirdas JT, Blake PG, Ing TS, editors. Hand book of Dialysis. Philadelphia: Lippincott Williams. 2007; 87-104.
- National Kidney Foundation. Updates Clinical Practice Guidelines and Recommendations. 2006.
- Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011 Mar;79(6):587-598. doi: 10.1038/ki.2010.471. Epub 2010 Dec 22. PMID: 21178979.
- Lata C, Girard L, Parkins M, James MT. Catheter-related bloodstream infection in end-stage kidney disease: a Canadian narrative review. Can J Kidney Health Dis. 2016 May 5;3:24. doi: 10.1186/s40697-016-0115-8. PMID: 27152201; PMCID: PMC4857243.
- Allon M. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am J Kidney Dis. 2004 Nov;44(5):779-91. PMID: 15492943.
- Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001 Jul;60(1):1-13. doi: 10.1046/j.1523-1755.2001.00765.x. PMID: 11422731.
- Beathard GA. Management of bacteremia associated with tunneled-cuffed hemodialysis catheters. J Am Soc Nephrol. 1999 May;10(5):1045-9. doi: 10.1681/ASN.V1051045. PMID: 10232691.
- Jaber BL. Bacterial infections in hemodialysis patients: pathogenesis and prevention. Kidney Int. 2005 Jun;67(6):2508-19. doi: 10.1111/j.1523-1755.2005.00364.x. PMID: 15882306.
- Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005 Sep;46(3):501-8. doi: 10.1053/j.ajkd.2005.05.024. PMID: 16129212.
- Weijmer MC, Vervloet MG, ter Wee PM. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant. 2004 Mar;19(3):670-7. doi: 10.1093/ndt/gfg581. PMID: 14767025.
- Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011 Mar;79(6):587-598. doi: 10.1038/ki.2010.471. Epub 2010 Dec 22. PMID: 21178979.
- Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, Allon M. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002 Mar;61(3):1136-42. doi: 10.1046/j.1523-1755.2002.00201.x. PMID: 11849468.
- Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteraemia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant. 2004 May;19(5):1237-44. doi: 10.1093/ndt/gfh041. Epub 2004 Feb 19. PMID: 14993504.
- Sychev D, Maya ID, Allon M. Clinical management of dialysis catheter-related bacteremia with concurrent exit-site infection. Semin Dial. 2011 Mar-Apr;24(2):239-41. doi: 10.1111/j.1525-139X.2011.00869.x. PMID: 21517993; PMCID: PMC4017937.
- Lisa MM, Edward C, Christine D, Swapnil H, Joanne K, Mercedeh K, Charmaine L, Rick L, Louise M, Matthew O, Jennifer M. Canadian Journal of Kidney Health and Disease. sagepub.com/journals. Permissions.nav. 2016; 3:1–11. DOI: 10.1177/205435811666912
- Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006 Jul;48 Suppl 1:S248-73. doi: 10.1053/j.ajkd.2006.04.040. PMID: 16813991.
- O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011 May;52(9):e162-93. doi: 10.1093/cid/cir257. Epub 2011 Apr 1. PMID: 21460264; PMCID: PMC3106269.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Jul 1;49(1):1-45. doi: 10.1086/599376. Erratum in: Clin Infect Dis. 2010 Apr 1;50(7):1079. Dosage error in article text. Erratum in: Clin Infect Dis. 2010 Feb 1;50(3):457. PMID: 19489710; PMCID: PMC4039170.
- Chan KE, Warren HS, Thadhani RI, Steele DJ, Hymes JL, Maddux FW, Hakim RM. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol. 2012 Sep;23(9):1551-9. doi: 10.1681/ASN.2012010050. Epub 2012 Aug 16. PMID: 22904350; PMCID: PMC3431413.
- Capdevila JA, Segarra A, Planes AM, Ramírez-Arellano M, Pahissa A, Piera L, Martínez-Vázquez JM. Successful treatment of haemodialysis catheter-related sepsis without catheter removal. Nephrol Dial Transplant. 1993;8(3):231-4. PMID: 8385290.
- Aslam S, Vaida F, Ritter M, Mehta RL. Systematic review and meta-analysis on management of hemodialysis catheter-related bacteremia. J Am Soc Nephrol. 2014 Dec;25(12):2927-41. doi: 10.1681/ASN.2013091009. Epub 2014 May 22. PMID: 24854263; PMCID: PMC4243345.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009 Jul 1;49(1):1-45. doi: 10.1086/599376. Erratum in: Clin Infect Dis. 2010 Apr 1;50(7):1079. Dosage error in article text. Erratum in: Clin Infect Dis. 2010 Feb 1;50(3):457. PMID: 19489710; PMCID: PMC4039170.
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, Craven DE; Infectious Diseases Society of America; American College of Critical Care Medicine; Society for Healthcare Epidemiology of America. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001 May 1;32(9):1249-72. doi: 10.1086/320001. Epub 2001 Apr 3. PMID: 11303260.
- Blot SI, Depuydt P, Annemans L, Benoit D, Hoste E, De Waele JJ, Decruyenaere J, Vogelaers D, Colardyn F, Vandewoude KH. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005 Dec 1;41(11):1591-8. doi: 10.1086/497833. Epub 2005 Oct 25. PMID: 16267731.
- Raad II, Hanna HA, Darouiche RO. Diagnosis of catheter-related bloodstream infections: is it necessary to culture the subcutaneous catheter segment? Eur J Clin Microbiol Infect Dis. 2001 Aug;20(8):566-8. doi: 10.1007/s100960100562. PMID: 11681436.
- Safdar N, Fine JP, Maki DG. Meta-analysis: methods for diagnosing intravascular device-related bloodstream infection. Ann Intern Med. 2005 Mar 15;142(6):451-66. doi: 10.7326/0003-4819-142-6-200503150-00011. Erratum in: Ann Intern Med. 2005 May 3;142(9):803. PMID: 15767623.
- Gaur AH, Flynn PM, Heine DJ, Giannini MA, Shenep JL, Hayden RT. Diagnosis of catheter-related bloodstream infections among pediatric oncology patients lacking a peripheral culture, using differential time to detection. Pediatr Infect Dis J. 2005 May;24(5):445-9. doi: 10.1097/01.inf.0000160950.83583.7f. PMID: 15876945.
- Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004 Jan 6;140(1):18-25. doi: 10.7326/0003-4819-140-1-200401060-00007. PMID: 14706968.
- Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007 Jul;51(7):2582-6. doi: 10.1128/AAC.00939-06. Epub 2007 Apr 23. PMID: 17452488; PMCID: PMC1913284.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004 Jun;42(6):2398-402. doi: 10.1128/JCM.42.6.2398-2402.2004. PMID: 15184410; PMCID: PMC427878.
- Lorente L, Jiménez A, Santana M, Iribarren JL, Jiménez JJ, Martín MM, Mora ML. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med. 2007 Oct;35(10):2424-7. doi: 10.1097/01.CCM.0000284589.63641.B8. PMID: 17717493.
- Reboli AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, Betts R, Wible M, Goldstein BP, Schranz J, Krause DS, Walsh TJ; Anidulafungin Study Group. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007 Jun 14;356(24):2472-82. doi: 10.1056/NEJMoa066906. PMID: 17568028.
- Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, Sekhon JS, Freire A, Ramasubramanian V, Demeyer I, Nucci M, Leelarasamee A, Jacobs F, Decruyenaere J, Pittet D, Ullmann AJ, Ostrosky-Zeichner L, Lortholary O, Koblinger S, Diekmann-Berndt H, Cornely OA; Micafungin Invasive Candidiasis Working Group. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007 May 5;369(9572):1519-1527. doi: 10.1016/S0140-6736(07)60605-9. PMID: 17482982.
- Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002 Jun;46(6):1773-80. doi: 10.1128/AAC.46.6.1773-1780.2002. PMID: 12019089; PMCID: PMC127206.
- Raad I, Hanna H, Boktour M, Girgawy E, Danawi H, Mardani M, Kontoyiannis D, Darouiche R, Hachem R, Bodey GP. Management of central venous catheters in patients with cancer and candidemia. Clin Infect Dis. 2004 Apr 15;38(8):1119-27. doi: 10.1086/382874. Epub 2004 Mar 26. Erratum in: Clin Infect Dis. 2004 Oct 15;39(8):1264. PMID: 15095217.
- Chee L, Brown M, Sasadeusz J, MacGregor L, Grigg AP. Gram-negative organisms predominate in Hickman line-related infections in non-neutropenic patients with hematological malignancies. J Infect. 2008 Apr;56(4):227-33. doi: 10.1016/j.jinf.2008.01.046. Epub 2008 Mar 17. PMID: 18342947.
- Rodríguez-Créixems M, Alcalá L, Muñoz P, Cercenado E, Vicente T, Bouza E. Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985-2006. Medicine (Baltimore). 2008 Jul;87(4):234-249. doi: 10.1097/MD.0b013e318182119b. PMID: 18626306.
- Blot S, Janssens R, Claeys G, Hoste E, Buyle F, De Waele JJ, Peleman R, Vogelaers D, Vandewoude K. Effect of fluconazole consumption on long-term trends in candidal ecology. J Antimicrob Chemother. 2006 Aug;58(2):474-7. doi: 10.1093/jac/dkl241. Epub 2006 Jun 6. PMID: 16757503.
- Doulton T, Sabharwal N, Cairns HS, Schelenz S, Eykyn S, O'Donnell P, Chambers J, Austen C, Goldsmith DJ. Infective endocarditis in dialysis patients: new challenges and old. Kidney Int. 2003 Aug;64(2):720-7. doi: 10.1046/j.1523-1755.2003.00136.x. PMID: 12846771.
- Shroff GR, Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients with bacterial endocarditis in the United States. Am J Kidney Dis. 2004 Dec;44(6):1077-82. doi: 10.1053/j.ajkd.2004.08.030. PMID: 15558529.
- National Council on Aging. 2002. Survey. 6: 11-18.
- American Diabetes Association. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes. 2018 Jan;36(1):14-37. doi: 10.2337/cd17-0119. PMID: 29382975; PMCID: PMC5775000.
- Daniel Pratt S. Evaluation of Liver Function. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL and Loscalzo J. Harrison’s Principles of Internal Medicine, 19th ed. New York, McGraw Hill: 2015; 1995 -1999.
- WHO. Hemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity, Vitamin and Mineral Nutrition Information System, World Health Organization, Geneva, Switzerland. 2011.
- Steven M, Holland H, John I, Gallin S. Disorders of Granulocytes and Monocytes. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL and Loscalzo J. Harrison’s Principles of Internal Medicine, 19th ed. New York, McGraw Hill. 2015; 413-423.
- Center for Disease Control and Prevention. Clinician Guide for Collecting Cultures. 2015.
- Fowler VG Jr, Justice A, Moore C, Benjamin DK Jr, Woods CW, Campbell S, Reller LB, Corey GR, Day NP, Peacock SJ. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis. 2005 Mar 1;40(5):695-703. doi: 10.1086/427806. Epub 2005 Feb 4. PMID: 15714415.
- Pigrau C, Rodríguez D, Planes AM, Almirante B, Larrosa N, Ribera E, Gavaldà J, Pahissa A. Management of catheter-related Staphylococcus aureus bacteremia: when may sonographic study be unnecessary? Eur J Clin Microbiol Infect Dis. 2003 Dec;22(12):713-9. doi: 10.1007/s10096-003-1041-0. Epub 2003 Nov 6. PMID: 14605943.
- Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J. 2004 Mar;147(3):536-9. doi: 10.1016/j.ahj.2003.09.018. PMID: 14999206.
- Vergis EN, Hayden MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Schmitt B, Muder RR. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Ann Intern Med. 2001 Oct 2;135(7):484-92. doi: 10.7326/0003-4819-135-7-200110020-00007. PMID: 11578151.
- Lorente L, Jiménez A, Santana M, Iribarren JL, Jiménez JJ, Martín MM, Mora ML. Microorganisms responsible for intravascular catheter-related bloodstream infection according to the catheter site. Crit Care Med. 2007 Oct;35(10):2424-7. doi: 10.1097/01.CCM.0000284589.63641.B8. PMID: 17717493.
- Chee L, Brown M, Sasadeusz J, MacGregor L, Grigg AP. Gram-negative organisms predominate in Hickman line-related infections in non-neutropenic patients with hematological malignancies. J Infect. 2008 Apr;56(4):227-33. doi: 10.1016/j.jinf.2008.01.046. Epub 2008 Mar 17. PMID: 18342947.
- Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006 Mar;47(3):469-77. doi: 10.1053/j.ajkd.2005.11.023. PMID: 16490626.
- Jaudah AK, Musa AK. Incidence and risk factors of central venous catheter and blood stream bacterial infections in hemodialysis patients: a cross sectional study. Life Science Archives (LSA). 2017; 3:1; 921-933.
- Farrington CA, Allon M. Complications of Hemodialysis Catheter Bloodstream Infections: Impact of Infecting Organism. Am J Nephrol. 2019;50(2):126-132. doi: 10.1159/000501357. Epub 2019 Jun 26. PMID: 31242483; PMCID: PMC6935870.
- Awad SM. Chronic renal failure in Al-Anbar of Iraq. Saudi J Kidney Dis Transpl. 2011 Nov;22(6):1280-4. PMID: 22089804.
- Malekmakan L, Haghpanah S, Pakfetrat M, Malekmakan A, Khajehdehi P. Causes of chronic renal failure among Iranian hemodialysis patients. Saudi J Kidney Dis Transpl. 2009 May;20(3):501-4. PMID: 19414964.
- Sanavi S, Ghods A, Afshar R. Catheter associated infections in hemodialysis patients. Saudi J Kidney Dis Transpl. 2007 Mar;18(1):43-6. PMID: 17237890.
- Samani S, Saffari M, Charkhchian M, Khaki A. Incidence and risk factors of bloodstream catheter-related infections in hemodialysis patients. Comparative Clinical Pathology. 2015 Mar 1; 24(2):275-9.
- Zanoni F, Pavone L, Binda V, Tripepi G, D'Arrigo G, Scalamogna A, Messa P. Catheter-related bloodstream infections in a nephrology unit: Analysis of patient- and catheter-associated risk factors. J Vasc Access. 2021 May;22(3):337-343. doi: 10.1177/1129729820939762. Epub 2020 Jul 10. PMID: 32648807.
- Hadian B, Zafarmohtashami A, Razani M. Catheter-related blood stream infections in hemodialysis patients. Journal of Renal Injury Prevention. 2020 Apr 23; 9(4):e34.
- Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011 Mar;79(6):587-598. doi: 10.1038/ki.2010.471. Epub 2010 Dec 22. PMID: 21178979.
- Lata C, Girard L, Parkins M, James MT. Catheter-related bloodstream infection in end-stage kidney disease: a Canadian narrative review. Can J Kidney Health Dis. 2016 May 5;3:24. doi: 10.1186/s40697-016-0115-8. PMID: 27152201; PMCID: PMC4857243.
- Thompson S, Wiebe N, Klarenbach S, Pelletier R, Hemmelgarn BR, Gill JS, Manns BJ, Tonelli M; Alberta Kidney Disease Network. Catheter-related blood stream infections in hemodialysis patients: a prospective cohort study. BMC Nephrol. 2017 Dec 8;18(1):357. doi: 10.1186/s12882-017-0773-5. PMID: 29221439; PMCID: PMC5723103.
- Farrington CA, Allon M. Management of the Hemodialysis Patient with Catheter-Related Bloodstream Infection. Clin J Am Soc Nephrol. 2019 Apr 5;14(4):611-613. doi: 10.2215/CJN.13171118. Epub 2019 Mar 5. PMID: 30837242; PMCID: PMC6450352.
- Mahmood SN, Asif S, Anwar MA, Naveed OK. Frequency and microbiological profile of catheter-related infections in hemodialysis patients receiving gentamicin as antimicrobial lock therapy for prophylaxis. Pakistan Journal of Kidney Diseases. 2020; 4(03).
- Sahli F, Feidjel R, Laalaoui R. Hemodialysis catheter-related infection: rates, risk factors and pathogens. J Infect Public Health. 2017 Jul-Aug;10(4):403-408. doi: 10.1016/j.jiph.2016.06.008. Epub 2016 Aug 8. PMID: 27423929.
- Powers AC. Diabetes Mellitus: Complications. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL and Loscalzo J. Harrison’s Principles of Internal Medicine, 19th ed. New York, McGraw Hill. 2015; 2422-2430.
- Ghonemy TA, Farag SE, Soliman SA, Amin EM, Zidan AA. Vascular access complications and risk factors in hemodialysis patients: A single center study. Alexandria Journal of Medicine. 2016 Mar 30; 52(1):67-71.
- Katneni R, Hedayati SS. Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat Clin Pract Nephrol. 2007 May;3(5):256-66. doi: 10.1038/ncpneph0447. PMID: 17457359.
- Leone S, Suter F. Severe bacterial infections in haemodialysis patients. Infez Med. 2010 Jun;18(2):79-85. PMID: 20610929.
- Gupta S, Mallya SP, Bhat A, Baliga S. Microbiology of Non-Tunnelled Catheter-Related Infections. J Clin Diagn Res. 2016 Jul;10(7):DC24-8. doi: 10.7860/JCDR/2016/19058.8155. Epub 2016 Jul 1. PMID: 27630843; PMCID: PMC5020248.
- Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011 Mar;79(6):587-598. doi: 10.1038/ki.2010.471. Epub 2010 Dec 22. PMID: 21178979.
- Daugirdos J1. Hand book of dialysis third edition, lippincott williams & wilkins, 2001; 4:43; 50:28:496-500.
- Labriola L. Antibiotic locks for the treatment of catheter-related blood stream infection: Still more hope than data. Semin Dial. 2019 Sep;32(5):402-405. doi: 10.1111/sdi.12807. Epub 2019 Apr 4. PMID: 30950116.