More Information
Submitted: February 26, 2022 | Approved: March 16, 2022 | Published: March 17, 2022
How to cite this article: Gupta KL, Bharati J. Community-acquired AKI and its management. J Clini Nephrol. 2022; 6: 026-029.
DOI: 10.29328/journal.jcn.1001086
Copyright License: © 2022 Gupta KL, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Community-acquired AKI and its management
Krishan Lal Gupta1* and Joyita Bharati2
and Joyita Bharati2
1Department of Nephrology, Sapthagiri Institute of Medical Sciences & Research Centre, Bangalore, India
2Assistant Professor, Department of Nephrology, Post Graduate Institute of Medical Education and Research, Chandigarh, India
*Address for Correspondence: Krishan Lal Gupta, Professor and Head, Department of Nephrology, Sapthagiri Institute of Medical Sciences & Research Centre, Bangalore, India, Email: [email protected]
Acute Kidney Injury (AKI) is defined as an abrupt decrease in kidney function within hours to days and is caused by multiple factors. Community-acquired AKI (CA-AKI) is common in developing countries, and it is crucial to bring awareness about its epidemiology and simple preventive strategies that can tackle this potentially serious complication. Infections, use of over-the-counter medicines, traditional herbal remedies, animal (and insect) bites, and pregnancy-related complications are common causes of CA-AKI in developing countries. The incidence of vector-borne disease-related AKI and obstetric causes of AKI have decreased following better public health policies in most developing countries. Appropriate fluid management is critical in AKI, both in terms of prevention of development and progression of AKI. Timely initiation and de-escalation of fluid therapy are both equally important. Kidney replacement therapy (KRT) is indicated when AKI progresses to stage 3 and/or patients develop refractory fluid overload or electrolyte imbalances and/or uremic complications. Hemodialysis is the most common modality of KRT in adults, whereas peritoneal dialysis is the dominant modality in small children. Convective renal replacement therapy, such as hemofiltration, is increasingly used in critically sick patients with AKI and hemodynamic instability. To summarize, CA-AKI is a common, serious, and often preventable complication of certain conditions acquired in the community, and is, therefore, a matter of utmost concern from the public health perspective.
Acute kidney injury (AKI) is a clinical syndrome due to an abrupt decrease in kidney function within hours to days. It is a heterogeneous disorder in terms of epidemiology, etiology, pathophysiology, and outcome. Historically, common causes of AKI throughout the globe occurred in the community, such as traumatic shock, infection, toxin exposure, and pregnancy complications. It was after World War II that reversibility of acute renal failure (ARF), particularly milder cases, was recognized widely. The change in the terminology of ARF to AKI in 2007, to include the complete spectrum of this disorder, highlights that a significant number of cases are mild (for example those acquired in the community) that are preventable and reversible. With economic progress, most preventable causes of community-acquired AKI (CA-AKI), such as tropical infections, snake bite toxin, and post-partum bleeding or septic abortion, have been effectively eliminated in developed countries. Therefore, CA-AKI due to the above-mentioned causes is largely restricted to developing countries in the current period.
AKI is defined as an increase in serum creatinine of ≥ 0.3 mg/dl over 48 hours or > 50% increase in serum creatinine over 7 days from baseline and/or oliguria i.e., urine output of < 0.5 ml/kg/hour over a 6-hour period at least [1]. Diagnosis of AKI requires information about baseline creatinine. However, AKI is diagnosed retrospectively in most cases of CA-AKI as confusion on baseline serum creatinine is common. Moreover, urine output is unknown at presentation as it is commonly not quantified, and getting ultrasonography for documenting kidney size and appearance has logistical issues in most first-contact hospitals in developing countries. Therefore, the practical implication of the existing AKI definitions, especially in the community setting, is limited.
Epidemiology and etiology
Reported incidence rates of AKI varies globally and is highly likely to be imprecise in developing countries due to multiple factors such as limited healthcare access and lack of electronic data capture system. Nevertheless, as per available data, the incidence of AKI in low-middle income countries is disproportionately higher (11.3 million cases per year) as compared to high-income countries (1.7 million cases per year) [2]. Although acute in nature, long-term development of chronic kidney disease(CKD),end-stage kidney disease (ESKD), and mortality are increased after an episode of AKI [3]. Epidemiology, adverse long-term impact, and economic loss associated with AKI are drivers for the foundation of the public health model of AKI [4]. The International Society of Nephrology (ISN) 0by25 initiatives aimed at targeting zero deaths from treatable AKI by 2025, focusing on the disadvantaged parts of Asia, Latin America, and Africa [5].
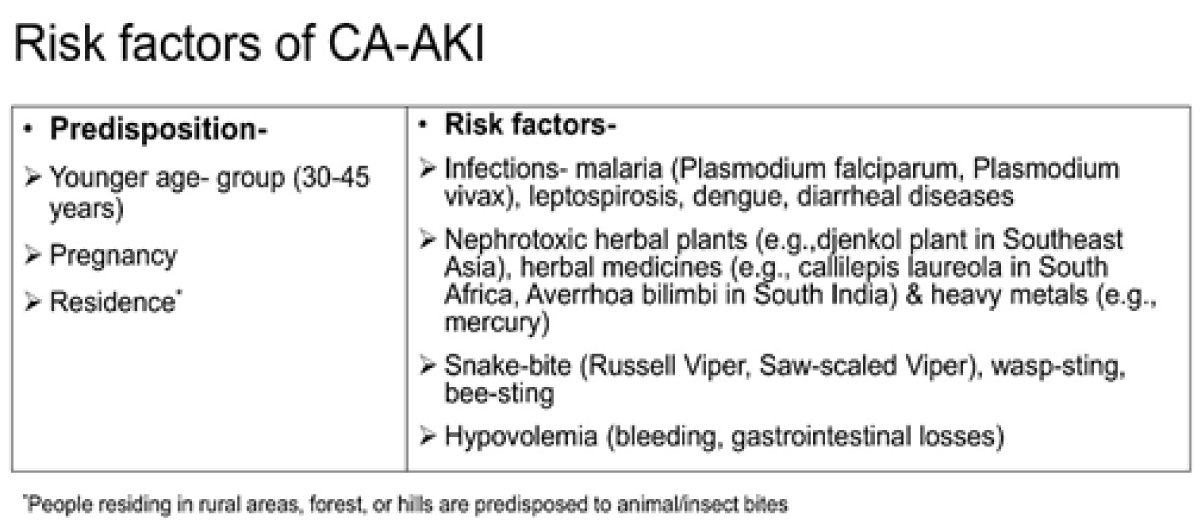
Common causes of CA-AKI in tropical (developing) countries are:
i) infections such as dengue viral infection, malaria, leptospirosis, scrub typhus, diarrheal illnesses, urosepsis, puerperal sepsis,
ii) ingestion of toxic herbal remedies such as toxic plants, heavy metals like mercury in alternative systems medicine,
iii) pregnancy-related complications such as postpartum hemorrhage, and
iv) animal/insect bites [6] (Figure 1).
Figure 1: Risk factors of community-acquired acute kidney injury.
CA-AKI outnumber hospital-acquired-AKIin developing countries, especially in children and women in rural areas, despite considerable economic growth [7]. More than half (58%) of the patients were observed to have CA-AKI in the Global Snapshot Study under the ISN 0by25 initiative, with 80% of these cases identified from the low-middle income countries [7]. Unlike hospital-acquired AKI which occurs commonly in the aging population with comorbidities, CA-AKI affects younger people (30-45 years of age) and often, has a single etiology [8]. While still common, the epidemiology of CA-AKI has been documented to have changed over past decades, with decreasing incidence of obstetrical AKI [9]. AKI related to diarrheal dehydration and septic abortion has been successfully tackled by most governments in the developing world [7], however, other causes of CA-AKI such as tropical infections, animal envenomation (snake bites in particular), nephrotoxic traditional remedies, and pregnancy-related complications such as antepartum hemorrhage and post-partum hemorrhage remain important public health challenges common to all developing countries [6,8]. A retrospective analysis of AKI epidemiology over 15 years in Pakistan showed the emergence of AKI due to toxic rhabdomyolysis from Paraphenylene diamine poisoning and dengue infections, respectively [10]. In a prospective observational study in a Southern state in India, acute pyelonephritis (17.7%) and snakebite (26.3%) were the most common causes of CA-AKI among 186 patients [11]. Another study from central India including 286 patients with CA-AKI reported medical (77.3%) causes to be the commonest followed by obstetrical (14%) and surgical (9%). Among medical causes of CA-AKI, hypo-perfusion and sepsis accounted for the majority and among obstetrical causes, puerperal sepsis accounted for the majority [12]. Poor socioeconomic status, inevitable interactions between rural population and flora and fauna of forests or fields which are a source of infective organisms and animal bites, type of climate, high dependency on non-standardized traditional remedies for treatment are some reasons for the rampancy of CA-AKI, particularly in the rural areas of developing countries [6].
Management
Disparate kidney pathologies are associated with CA-AKI requiring different approaches of management. On one hand, a significant number of cases are a consequence of non-specific conditions such as infections and dehydration resulting in acute tubular necrosis (acute cortical necrosis, in its extreme form) which are best observed for spontaneous recovery, in addition to treating the underlying cause with antibiotics, anti-venom, blood product transfusion, etc. On the other hand, a good number of cases of CA-AKI in developing countries are due to specific kidney pathologies such as acute tubule-interstitial nephritis, thrombotic microangiopathy, and acute glomerulonephritis which need specialist care, urine microscopy, and kidney biopsy as essential diagnostic tools. These conditions would need to be treated with high-end therapies such as plasmapheresis, immunosuppressive therapies, etc.
Prevention: Early recognition and prompt management of conditions causing AKIare crucial at the community level. Improving the healthcare work force [13] and increasing awareness would facilitate access to timely treatment. Simple and comprehensive protocols to manage common community-acquired illnesses such as tropical infections, snake bites, pregnancy-related complications, and dehydration would improve the outcome of CA-AKI.
Fluid therapy in the management of AKI: Timely fluid therapy, in the form of resuscitation, is crucial to prevent AKI in states of hypovolemia, contrast infusion, rhabdomyolysis, and post-surgery. However, excess fluid therapy or omission of fluid de-escalation in patients with early AKI is associated with less renal recovery [14]. Similarly, fluid overload in patients starting on kidney replacement therapy is associated with less renal recovery in the long term [15] and excess mortality. Therefore, fluids are indicated only when patients show fluid responsiveness, such as improved heart rate, skin turgor, blood pressure, or urine output. Oliguria itself is not an ideal parameter for assessing fluid responsiveness, particularly in patients with established AKI where oliguria does not necessarily reflect renal hypoperfusion.
Diuretic therapy in the management of AKI: Loop diuretics are used in the management of AKI for increasing tubular filtrate flow and controlling hypervolemia. The use of loop diuretics to prevent or treat AKI in terms of kidney outcome parameters is not recommended as renal hypoperfusion from hypovolemia may sustain or worsen AKI [16]. A Loop diuretic is also used to predict the prognosis of AKI in the form of the “frusemide challenge test”.
Kidney replacement therapy in AKI: Kidney replacement therapy, in the form of intermittent hemodialysis, peritoneal dialysis, continuous renal replacement therapy (CRRT), is used to treat severe AKI presenting with symptoms attributable to uremia. Peritoneal dialysis is the predominant modality used in smaller children with AKI due to the ease of the procedure and difficulty in securing vascular access required for hemodialysis. Most trials comparing the timing of initiation of KRT have failed to show superiority of early initiation as compared to delayed initiation when symptoms develop indicating urgent dialysis [17]. However, the threshold for initiating dialysis in some groups of patients such as tumor lysis syndrome, rhabdomyolysis, and critically sick patients requiring fluid space for nutrition and other drugs is lower. Although there are no clinical studies that have shown the superiority of CRRT when compared to intermitted slow low-efficiency dialysis in patients with AKI, CRRT is commonly the preferred modality in critically sick patients with hemodynamic instability.
Patient outcomes
Long-standing and severe AKI at presentation are associated with poor renal recovery and survival [2]. Absence of specific symptoms early in the course of AKI coupled with a lack of awareness about kidney diseases result in late diagnosis of AKI in developing countries.
In a prospective study at PGIMER Chandigarh (un-published data), urinary biomarkers measured at discharge were found to be associated with non-recovery of kidney function at 4 months after discharge in patients admitted with CA-AKI. Of a total of 78 patients with CA-AKI admitted to the hospital, 96% had stage 3 AKI and 27% had no recovery of renal function (defined as eGFR (CKD-EPI) > 60 ml/min/1.73m2 and urine protein creatinine ratio < 500 mg/g at 4 months). Amongst multiple urinary biomarkers, i.e., liver type-fatty acid-binding protein (L-FABP), neutrophil gelatinase-associated lipocalin (NGAL) (17), KIM-1(Kidney Injury Molecule -1), urinary NGAL, and KIM-1 (measured at discharge) were observed to be significantly low in patients with no renal recovery at 4 months after discharge. Urinary NGAL and uKIM-1, individually predicted failure to recover renal function at 4 months after CA-AKI with the area under the curve of 0.71 (95% CI, 0.26 to 0.55) and 0.68 (95% CI, 0.52 to 0.84), respectively. Such prospective studies using novel biomarkers are crucial in risk estimation after CA-AKI and appropriate follow-up.
Mortality rates in patients with AKI in low-middle income countries are reported to be associated with the income status of the country and healthcare accessibility [5]. Children with AKI in the low-middle-income countries were observed to have a 57-fold higher risk of death as compared to contemporaries from the high-income countries [18]. On the contrary, recovery of AKI is observed to be better in patients in low-middle-income countries compared to high-income countries [5]. Factors such as the absence of comorbidities in the affected younger patient population and distinct causes of AKI (CA-AKI) are speculated to be driving the favorable recovery of AKI in developing countries. However, these findings have not been formally tested in the form of a study. Long-term cohort studies on consequences of AKI such as CKD, ESKD, cardiovascular diseases, and long-term mortality are reported exclusively from high-income countries [19].
CA-AKI is often a preventable complication of conditions of public health concern in developing countries. With concerted efforts by governments, obstetrical causes of AKI, such as septic abortion, and vector-borne disease-related AKI, such as malaria, have substantially been controlled in most countries. Conventional definitions used to diagnose AKI have limited practical implications for patients with CA-AKI. Judicious fluid therapy is critical in preventing complications of AKI and overall mortality. Diuretic use to prevent AKI is only limited to certain patient populations. Hypervolemia control is the only indication for diuretic use in patients with established AKI. While KRT is commonly initiated after symptoms attributed to uremia develop in patients with severe AKI, individual cases with defined risk factors need early KRT initiation.
- Thomas ME, Blaine C, Dawnay A, Devonald MAJ, Ftouh S, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015; 87: 62–73. PubMed: https://pubmed.ncbi.nlm.nih.gov/25317932/
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013; 8: 1482–1493. PubMed: https://pubmed.ncbi.nlm.nih.gov/23744003/
- Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012; 81: 442–448. PubMed: https://pubmed.ncbi.nlm.nih.gov/22113526/
- Kam Tao Li P, Burdmann EA, Mehta RL, World Kidney Day Steering Committee 2013. Acute kidney injury: Global health alert. J Nephropathol. 2013; 2: 90–97. PubMed: https://pubmed.ncbi.nlm.nih.gov/23302721/
- Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015; 385: 2616–2643. PubMed: https://pubmed.ncbi.nlm.nih.gov/25777661/
- Kumar V, Jha V. Community-Acquired AKI in Asia: An Update. Semin Nephrol. 2020; 40: 456–467. PubMed: https://pubmed.ncbi.nlm.nih.gov/18620956/
- Mehta RL, Burdmann EA, Cerdá J, Feehally J, Finkelstein F, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016; 387: 2017–2025. PubMed: https://pubmed.ncbi.nlm.nih.gov/27086173/
- Jha V, Parameswaran S. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013; 9: 278-290. PubMed: https://pubmed.ncbi.nlm.nih.gov/23458924/
- Prakash J, Pant P, Prakash S, Vohra R, Doley PK, et al. Changing picture of acute kidney injury in pregnancy: Study of 259 cases over a period of 33 years. Indian J Nephrol. 2016; 26: 262-267. PubMed: https://pubmed.ncbi.nlm.nih.gov/27512298/
- Naqvi R. Epidemiological trends in community acquired acute kidney injury in Pakistan: 25 years Experience from a Tertiary Care Renal Unit. Pak J Med Sci. 2021: 27: 312-319. PubMed: https://pubmed.ncbi.nlm.nih.gov/33679905/
- Kaaviya R, Vadivelan M, Balamurugan N, Parameswaran S, Thabah MM. Community acquired AKI: A prospective observational study from a tertiary level hospital in Southern India. Indian J Nephrol. 2019; 29: 254-260. PubMed: https://pubmed.ncbi.nlm.nih.gov/31423059/
- Goswami S, Raju BM, Purohit A, Pahwa N. Clinical spectrum of community-acquired acute kidney injury: A prospective study from central India. Saudi J Kidney Dis Transpl. 2020; 31: 224-234. PubMed: https://pubmed.ncbi.nlm.nih.gov/32129217/
- Riaz P, Caskey F, McIsaac M, Davids R, Htay H, et al. Workforce capacity for the care of patients with kidney failure across world countries and regions. BMJ Glob Health. 2021; 6.
- Raimundo M, Crichton S, Martin JR, Syed Y, Varrier M, et al. Increased Fluid Administration After Early Acute Kidney Injury is Associated with Less Renal Recovery. Shock. 2015; 44: 431-437. PubMed: https://pubmed.ncbi.nlm.nih.gov/26263435/
- Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, et al. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012; 27: 956-9561. PubMed: https://pubmed.ncbi.nlm.nih.gov/21856761/
- Patschan D, Patschan S, Buschmann I, Ritter O. Loop Diuretics in Acute Kidney Injury Prevention, Therapy, and Risk Stratification. Kidney Blood Press Res. 2019; 44: 457-464. PubMed: https://pubmed.ncbi.nlm.nih.gov/31362295/
- Sohaney R, Yessayan LT, Heung M. Towards Consensus in Timing of Kidney Replacement Therapy for Acute Kidney Injury? Am J Kidney Dis. 2021; 77: 542-545. PubMed: https://pubmed.ncbi.nlm.nih.gov/32920155/
- Macedo E, Cerdá J, Hingorani S, Hou J, Bagga A, et al. Recognition and management of acute kidney injury in children: The ISN 0by25 Global Snapshot study. PloS One. 2018; 13: e0196586. PubMed: https://pubmed.ncbi.nlm.nih.gov/29715307/
- See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019; 95: 160-172. PubMed: https://pubmed.ncbi.nlm.nih.gov/30473140/