More Information
Submitted: June 22, 2021 | Approved: July 22, 2021 | Published: July 23, 2021
How to cite this article: Faizan MK, McCracken C, Lieberman K, Leong T, Benfield MR. Practice patterns and outcomes of repository corticotropin injection (Acthar® Gel) use in childhood nephrotic syndrome: A study of the North American Pediatric Renal Trials and collaborative studies and the Pediatric Nephrology Research Consortium. J Clini Nephrol. 2021; 5: 067-076.
DOI: 10.29328/journal.jcn.1001077
Copyright License: © 2021 Faizan MK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nephrotic syndrome; Children; Adrenocorticotropic hormone; Registry
Abbreviations: CR: Complete Remission; PR: Partial Remission; NR: No Remission; FSGS: Focal Segmental Glomerulosclerosis; IgAN: IgA Nephropathy; MCD: Minimal Change Disease; MN: Membranous Nephropathy; MPGN: Membranoproliferative Glomerulonephritis; SLE: Systemic Lupus Erythematosus; rFSGS: post-transplant recurrent FSGS
Practice patterns and outcomes of repository corticotropin injection (Acthar® Gel) use in childhood nephrotic syndrome: A study of the North American Pediatric Renal Trials and collaborative studies and the Pediatric Nephrology Research Consortium
Mohammed K Faizan1*, Courtney McCracken2, Kenneth Lieberman3, Traci Leong2 and Mark R Benfield4
1Hasbro Children’s Hospital, Providence, RI, USA
2Emory University and Children’s Healthcare of Atlanta, Atlanta, GA, USA
3Hackensack Meridian Health, Hackensack, NJ, USA
4Pediatric Nephrology of Alabama, PC, Birmingham, AL, USA
*Address for Correspondence: Mohammed K Faizan, Hasbro Children’s Hospital, Providence, RI, USA, Email: [email protected]
Objective: We set up a U.S. registry to examine prescription patterns and patient outcomes of repository corticotropin injection (Acthar® Gel) for childhood nephrotic syndrome.
Methods: 18 participating U.S. pediatric centers performed retrospective review and prospective observation of patients < 21 years old with nephrotic syndrome treated with Acthar Gel. We captured baseline characteristics, drug regimen and duration, and disease response following treatment.
Results: 46 patients, enrolled from 2015 to 2020 were included. 27 (58.7%) were male. 18 patients (39.1%) had a diagnosis of minimal change followed by focal segmental glomerulosclerosis in 16 patients (34.7%). Median age at start of treatment was 12.5 years (IQR 8.5-17.4) compared to 5.3 years at diagnosis (IQR 2.7-10.5 years). 52% were resistant to corticosteroids. The most common Acthar Gel regimen was 80IU twice a week with a median duration of 199 days (IQR 88-365). Among 37 patients with active disease, 18 (49%) were able to achieve partial or complete remission, though all patients that had a positive response were on other immunosuppressants concomitantly.
Conclusion: We report the findings of the largest registry cohort of pediatric patients in the U.S. treated with Acthar Gel for clinically challenging cases of nephrotic syndrome. Acthar Gel was successful in inducing remission in approximately half of the patients with active disease at time of treatment. No predictors of response with respect to demographic data, age at start of Acthar Gel therapy, etiology of nephrotic syndrome, presence or absence of comorbidities, or steroid responsiveness was noted.
Treatment of nephrotic syndrome (NS) in children remains challenging. High dose corticosteroids and other immunosuppressants have significant side effects that limit their tolerability [1, 2]. Moreover, treatment resistant NS carries a high risk for progression to end-stage kidney disease (ESKD) [3]. Effective therapeutic agents with low side-effect profile are urgently needed.
Adrenocorticotropic hormone (ACTH) was one of the earliest drugs used for NS treatment. Used widely in the 1950s for treatment naïve pediatric patients, ACTH induced NS disease remission and significantly reduced disease mortality [4]. In the 1960s, ACTH, administered via injections, was replaced by oral prednisone due to its ease of administration and the belief that ACTH exerted its effects mainly through steroidogenesis [5]. In recent years, there is renewed interest in ACTH for the treatment of NS, stemming from adult studies demonstrating its ability to induce remission and improve serum lipid profile in steroid- and multidrug-resistant NS with minimal side effects, suggestive of additional effects beyond steroidogenesis, though that hypothesis remains unproven [6-9]. In the United States (U.S.), ACTH is now available as a purified repository gel, Acthar® Gel (Mallinckrodt Pharmaceuticals), given most commonly in adults at 80U twice weekly for six months [5]. Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone analogs and other pituitary peptides. The manufacturing process converts the initial porcine pituitary extract with low ACTH content into a mixture having modified porcine ACTH and other related peptide analogs solubilized in gelatin. A major component in the formulated complex mixture is N-25 deamidated porcine ACTH(1-39) [10]. Acthar Gel dosing and regimen cannot be directly compared with published experiences in the 1950s, or reports from Europe, due to differences in formulations. There is minimal published experience on Acthar Gel use in pediatric patients with NS apart from a recently published clinical trial in steroid-sensitive children with NS (ATLANTIS, ClinicalTrials.gov Identifier: NCT02132195) [11].
We aimed to systematically collect practice patterns and treatment outcomes of contemporary Acthar Gel use in pediatric patients with NS and set up a data registry through the networks of two large international research collaborations, the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) and the Pediatric Nephrology Research Consortium (PNRC). To our knowledge, this is the largest case series of pediatric NS patients treated with Acthar Gel.
Acthar® Gel Registry is an observational data registry started in 2015 among 18 voluntary U.S. centers, recruited through NAPRTCS and PNRC. For this analysis, patients enrolled through 2018 were included. Both NAPRTCS and PNRC are pediatric nephrology research networks that are comprised of 120 centers and 80 centers, respectively. Inclusion Criteria: All patients treated with Acthar Gel between age 6 months to 21 years for clinically diagnosed nephrotic syndrome nephrotic range proteinuria with urine protein to creatinine ratio [UPCr] > 2 mg/mg or 24 hour urine protein > 3 g, hypoalbuminemia with serum albumin ≤ 2.5 g/dL, and edema) were approached for consent for registry enrollment.
Data collection
Demographic and clinical information were collected both retrospectively via chart review and prospectively. Baseline (Time 0) was defined as the date of the first dose of Acthar Gel. Demographic characteristics captured included: age (at time of diagnosis and at start of Acthar Gel), gender, race, and ethnicity. Clinical characteristics captured included: current disease status (active disease defined as UPCr > 2 mg/mg, low serum albumin, or edema vs. disease remission defined as UPCr ≤ 0.5 mg/mg), Acthar Gel dose and frequency, reasons for starting Acthar Gel, histologic diagnosis (minimal change disease [MCD], focal segmental glomerulosclerosis [FSGS], membranoproliferative glomerulonephritis [MPGN], IgA nephropathy [IgAN], membranous nephropathy [MN], lupus nephropathy [LN], or other), steroid response pattern (steroid sensitive nephrotic syndrome [SSNS], achieve remission within 4 weeks of daily prednisone/prednisolone therapy; steroid dependent [SDNS], relapses during corticosteroid taper or within 2 weeks after discontinuing corticosteroids; or steroid resistant [SRNS], persistent proteinuria despite 4 weeks of daily corticosteroid therapy), relapse frequency (infrequent, <4 relapses a year; frequent, ≥ 4 relapses a year), history of renal replacement therapy (RRT), history of renal transplant, and co-morbid conditions (i.e. asthma, epilepsy, etc.). Previous and current therapies for NS treatment, including duration and dose, were also recorded (methylprednisolone, cyclophosphamide, cyclosporine, rituximab, mycophenolate, tacrolimus, azathioprine, and angiotensin-converting-enzyme inhibitors [ACEi]). Laboratory results in the 3 months prior to Acthar Gel initiation were captured, including: serum creatinine, serum albumin, and UPCr. Information on underlying genetic diagnoses as a possible cause of their NS was not collected. In addition, food intake was also not recorded.
Clinical information following Acthar Gel initiation (Time 0) were captured at 1 month and then every 6 months of use. Data captured included: Acthar Gel dosing change and duration, number of disease relapses and time to relapse (if applicable), reported side effects of Acthar Gel, disease complications (i.e. progression to ESKD, infections requiring antimicrobial treatment, blood clots, etc.), concomitant medications, and laboratory results (serum creatinine, serum albumin, and UPCr).
Treatment outcome follow-up
Clinical response to Acthar Gel for patients with active disease at the start of Acthar Gel was defined by the degree of proteinuria, serum albumin, and presence of edema during Acthar Gel treatment. Response was classified as: complete response (CR) - serum albumin ≥ 3.5 g/dL, UPCr < 0.5 mg/mg, and no edema; partial response (PR) - an increase in serum albumin > 0.5 g/dL and decrease in UPCr by > 50%; and no response (NR) - worsening or no improvements in serum albumin > 0.5 g/dL or UPCr > 50%. For patients who were in disease remission at the start of Acthar Gel treatment, clinical response to Acthar Gel was examined by time-to-subsequent relapse (if applicable) and the number of disease relapses during Acthar Gel treatment.
Statisical analysis
Patient and Acthar Gel regimen characteristics were summarized using means and standard deviations (SDs), medians and interquartile ranges (IQRs), and counts and percentages, where appropriate. Duration of Acthar Gel treatment was displayed graphically using a Kaplan Meier plot.
Acthar Gel treatment response was described as follows. For patients with active disease at the start of Acthar Gel (majority of patients, 37 out of 46), response to Acthar Gel by disease histologic classification was summarized graphically, and potential associations with baseline characteristics were explored using Chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. All statistical analyses were performed using SAS Version 9.4 (Cary, NC) and statistical significance was assessed at the 0.05 level, unless otherwise noted.
Description of the registry cohort
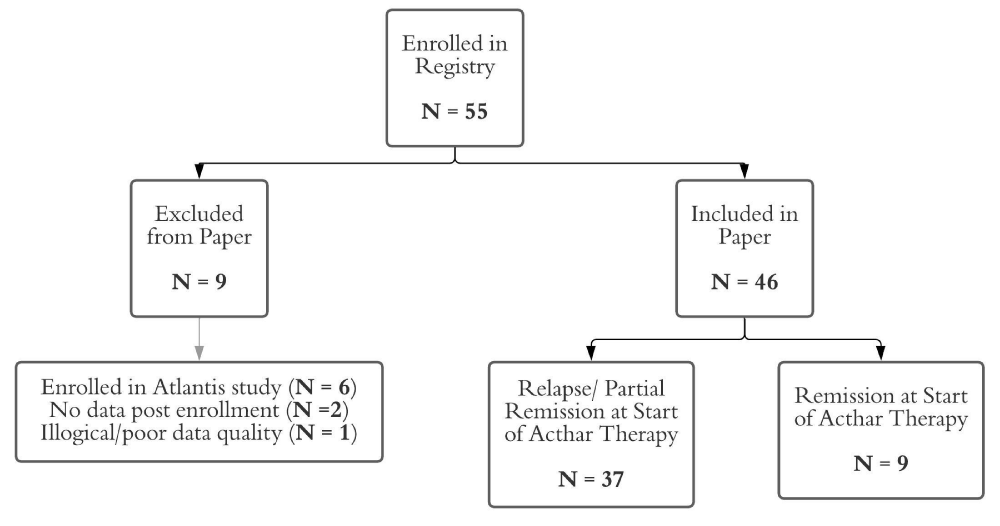
A total of 55 patients were enrolled in the Acthar Gel Registry between April 1, 2015 to December 1, 2020; of which 6 patients were excluded due to participation in a clinical trial of Acthar Gel use (ATLANTIS) and 3 due to lack of data or poor data quality (Figure 1). Forty-six patients were analyzed for this report; their demographic and clinical characteristics at the start of Acthar Gel are described in table 1. Eighteen patients (39.1%) had a diagnosis of minimal change followed by focal segmental glomerulosclerosis in 16 patients (34.7%). The remaining patients had less common causes of pediatric nephrotic syndrome including IgAN [2/46, 4.4%], MN [2/46, 4.4%], MPGN [2/46, 4.4%], SLE [2/46, 4.45], and 1 patient had no biopsy [1/46, 2.2%]. Three patients (6.5%) had post-transplant recurrent FSGS. On average, the cohort began Acthar Gel well after initial diagnosis with a median age at therapy start of 12.5 years (IQR 8.5-17.4) compared to median age at diagnosis of 5.3 years (IQR 2.7-10.5). A large proportion were resistant to corticosteroids [24/46, 52.2%], had active disease as indicated by UPCr > 2 mg/mg [37/46, 80.4%], or had serious complications such as hypertension (19/46, 41%) or acute kidney injury requiring renal replacement therapy (3/46, 7%). Two patients, with MN and MPGN, were not previously treated with corticosteroids. All but two patients (97%) had been treated with at least one immunosuppressant that was not corticosteroids, with a median of 3 prior therapies (IQR, 2-4).
| Table 1: Demographic and clinical characteristics of 46 pediatric patients with nephrotic syndrome enrolled in the Acthar Gel Registry from 2015-2020 by disease statusa at start of Acthar Gel therapy. | |||
| Characteristic | Total (n = 46) | Active Disease (N = 37) | Remission (n = 9) |
| Male—n (%) | 27 (58.7%) | 22 (59.5%) | 5 (55.6%) |
| Race—n (%) | |||
| White | 29 (63.0%) | 23 (62.2%) | 6 (66.7%) |
| Black | 7 (15.2%) | 5 (13.5%) | 2 (22.2%) |
| Asian | 4 (8.7%) | 4 (10.8%) | 0 (0%) |
| Other | 4 (8.7%) | 3 (8.1%) | 1 (11.1%) |
| Unknown | 2 (4.4%) | 2 (5.4%) | 0 (0%) |
| Hispanic Ethnicity—n (%) | 12 (26.1%) | 10 (27.0%) | 2 (22.2%) |
| Age at Diagnosis--yr, median (IQR), [min, max] | 5.3 (2.7 - 10.5) [0.8, 19.2] |
5.3 (2.6 – 10.3) [0.8, 19.2] |
4.9 (4.0 – 12.8) [2.6, 17.0] |
| Age at Acthar® Gel Start--yr, median (IQR), [min, max] | 12.5 (8.5 – 17.4) [4.2, 20.3] |
12.5 (8.6 – 16.6) [4.2, 20.2] |
15.4 (7.6 – 17.4) [6.2, 20.3] |
| Diagnosis—n (%) | |||
| MCD | 18 (39.1%) | 14 (37.8%) | 4 (44.4%) |
| FSGS | 16 (34.7%) | 12 (32.4%) | 4 (44.4%) |
| IgAN | 2 (4.4%) | 2 (5.4%) | 0 (0%) |
| MPGN | 2 (4.4%) | 1 (2.7%) | 1 (11.1%) |
| MN | 2 (4.4%) | 2 (5.4%) | 0 (0%) |
| SLE | 2 (4.4%) | 2 (5.4%) | 0 (0%) |
| No biopsy | 1 (2.2%) | 1 (2.7%) | 0 (0%) |
| Post-Transplant Recurrent FSGS | 3 (6.5%) | 3 (8.1%) | 0 (0%) |
| Steroid Response Classification—n (%) | |||
| Steroid Responsive | 6 (13.0%) | 5 (13.5%) | 1 (11.1%) |
| Steroid Dependent | 14 (30.4%) | 9 (24.3%)) | 5 (55.6%) |
| Steroid Resistant | 24 (52.2%) | 22 (59.5%) | 2 (22.2%) |
| No Previous Steroid Therapy | 2 (4.4%) | 1 (2.7%) | 1 (11.1%) |
| History of Dialysis—n (%) | 3 (6.5%) | 3 (8.1%) | 0 (0%) |
| Transplant Recipient—n (%) | 3 (6.5%) | 3 (8.1%) | 0 (0%) |
| Diagnosis of Hypertension—n (%) | 19 (41.3%) | 14 (37.8%) | 5 (55.6%) |
| Number of Previous ISTsb—n, median (IQR), [min, max] | 3 (2 – 4) [0, 7] |
2 (2 – 4) [0, 7] |
4 (3 – 5) [1, 6] |
| Prior ISTsb—n (%) | |||
| Solumedrol | 20 (43.5%) | 14 (37.8%) | 6 (66.7%) |
| Cyclophosphamide | 13 (28.3%) | 9 (24.3%) | 4 (44.4%) |
| Cyclosporine | 14 (30.4%) | 11 (29.7%) | 3 (33.3%) |
| Rituximab | 15 (32.6%) | 10 (27.0%) | 5 (55.6%) |
| Mycophenolate Mofetil | 25 (54.4%) | 20 (54.1%) | 5 (55.6%) |
| Tacrolimus | 28 (60.9%) | 21 (56.8%) | 7 (77.8%) |
| Azathioprine | 3 (6.5%) | 3 (8.1%) | 0 (0%) |
| ACEi/ARB for antiproteinuric effect | 26 (56.5%) | 23 (62.2%) | 3 (33.3%) |
| UPCr—mg/mg, median (IQR) (n = 34) | 3.5 (1.1 – 7.4) | 4.71 (2.13 – 9.46) | 0.12 (0.08 – 0.60) |
| Estimate GFRc, median (IQR) (n = 43) | 101.7 (66.8 – 128.9) |
102.3 (64.7 – 128.9) |
97.7 (90.3 – 111.5) |
| Serum creatinine—mg/dL, median (IQR) (n = 43) | 0.60 (0.50 – 0.82) |
0.60 (0.50 – 0.82) |
0.71 (0.50 – 0.75) |
| aDisease status defined as: active disease (UPCr > 2 mg/mg, low albumin, or edema) vs. remission (UPCr ≤ 0.5 mg/mg) bNot including oral corticosteroids. cEstimated GFR is calculated from serum creatinine at enrollment using the Beside Schwartz formula [16] Abbreviations: ACEi: Angiotensin-Converting-Enzyme inhibitors; eGFR: estimated Glomerular Filtration Rate; FSGS: Focal Segmental Glomerulosclerosis; IgAN: IgA Nephropathy; IQR: Interquartile Range; IST: Immunosuppressive Therapy; IV: Intravenous; yr: year; MCD: Minimal Change Disease; MN: Membranous Nephropathy; MPGN: Membranoproliferative Glomerulonephritis; NS: Nephrotic Syndrome; SLE: Systemic Lupus Erythematosus; SSNS: Steroid-Sensitive Nephrotic Syndrome; SRNS: Steroid-Resistant Nephrotic Syndrome; UPCr: Urine Protein to Creatinine ratio |
|||
Administration of Acthar Gel
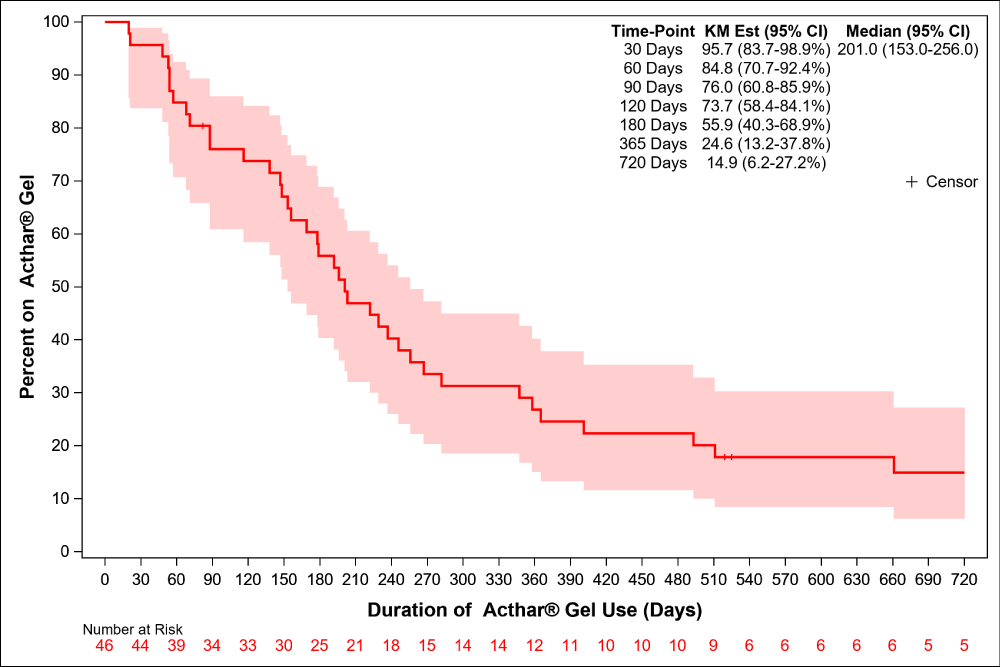
As stated above, in 37/46 (80.4%) of patients, Acthar Gel was started during active disease (as indicated by UPCr > 2 mg/mg, low albumin or edema) and the remaining 9 patients (19.6%) were in remission with UPCr < 0.5 mg/mg (Figure 1). The starting dose, frequency of administration, whether or not doses were escalated, target dose, and dose frequency are described in Table 2. The most common treatment regimen, used in over half of the patients [27/46, 58.7%] was 80IU twice weekly, though variations exist. The median duration of Acthar Gel treatment was 199 days, with a large variation of treatment duration (IQR 88-365). Figure 2 shows the proportion of patients taking Acthar Gel over time. At the start of Acthar Gel treatment, 28/46 (61%) patients were concomitantly treated with immunosuppressants other than oral corticosteroids, with a majority of patients continuing on their immunosuppressants while taking Acthar Gel (Table 2).
Figure 1: Description of Registry Cohort.
Figure 2: Percent of patients on Acthar Gel over time.
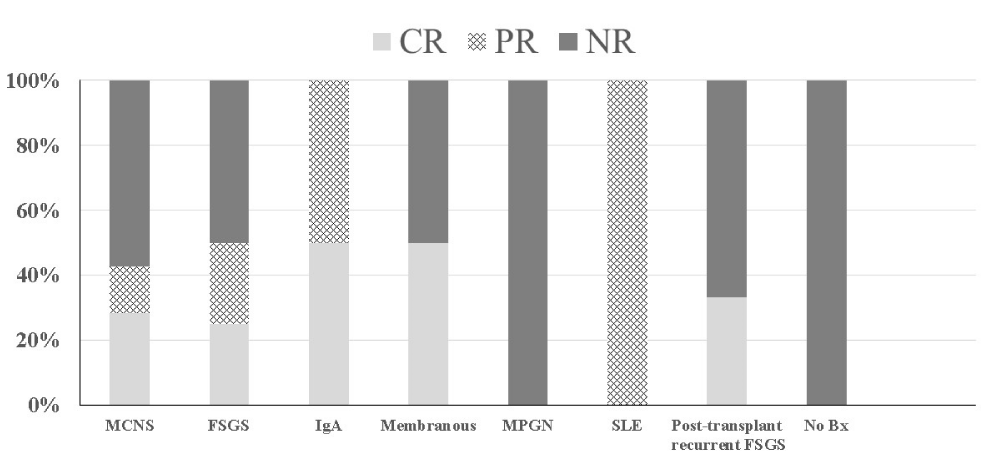
Among the 37 patients who had active disease with nephrotic range proteinuria at the start of Acthar Gel, 10 (27%) achieved a complete remission (CR) and 8 (21%) reached partial remission (PR) during Acthar Gel treatment. The median duration of treatment among those who achieved either CR or PR remission was 192 days (IQR 116-347). Nineteen patients (51%) failed to achieve partial or complete remission on Acthar Gel, with median duration of treatment of 148 days (IQR 80 - 198). The treatment response among the 37 patients with active disease by histologic diagnosis is presented in Figure 3.
Figure 3: Response to Acthar Gel among 37 pediatric patients with active nephrotic syndrome at start of treatment by disease histology.
| Table 2: Acthar Gel prescription among 46 pediatric patients with nephrotic syndrome by disease statusa at start of Acthar Gel therapy. | |||
| Prescription Characteristics | Total (n = 46) | Active Disease (N = 37) |
Remission (N = 9) |
| Starting Dose—IU, median (IQR), [min, max] | 44 (40 – 80) [20,80] |
48 (40 – 80) [20, 80] |
40 (40 – 80) [20, 80] |
| Starting Dose per BSA—IU/m2, median (IQR), [min, max] | 38.4 (26.0, 46.7) [18.8, 121.2] |
37.6 (26.0 – 46.7) [18.8, 121.2] |
39.6 (27.0 – 45.9) [20.3, 57.3] |
| Starting Frequency—n (%) | |||
| 1/week | 2 (4.4%) | 2 (5.4%) | 0 (0%) |
| 2/week | 30 (65.2%) | 24 (64.9%) | 6 (66.7%) |
| 3/week | 14 (30.4%) | 11 (29,7%) | 3 (33.3%) |
| Dose Escalated to Reach Target—n (%) | 23 (50.0%) | 16 (43.2%) | 7 (77.8%) |
| Target Dose—IU, median (IQR), [min, max] | 80 (80 – 80) (35, 80) |
80 (64 – 80) [40, 80] |
80 (80 – 80) [35, 80] |
| Target Dose per BSA—IU/m2, median (IQR), [min, max] | 48.5 (43.0 – 71.5) [37.6, 81.7] |
49.8 (42.3, 55.9) [37.6, 81.7] |
47.3 (45.9, 73.2) [40.6, 79.2] |
| Target Frequency—n (%) | |||
| 2/week | 15 (65.2%) | 11 (68.8%) | 4 (57.1%) |
| 3/week | 7 (30.4%) | 5 (31.3%) | 2 (28.6%) |
| Other | 1 (4.4%) | 0 (0%) | 1 (14.3%) |
| Treatment Duration—days, median (IQR) | 199 (88 – 365) | 192 (116 – 347) | 256 (57 – 519) |
| Concomitant Therapy—n (%) | |||
| Oral corticosteroids | 28 (60.9%) | 24 (64.9%) | 4 (44.4%) |
| Cyclosporine | 6 (13.0%) | 5 (13.5%) | 1 (11.1%) |
| Mycophenolate Mofetil | 13 (28.3%) | 11 (29.7%) | 2 (22.2%) |
| Tacrolimus | 18 (39.1%) | 13 (35.1%) | 5 (55.6%) |
| ACEi or ARB | 29 (63.0%) | 22 (59.5%) | 7 (77.8%) |
| Reason for Stopping Acthar® Gel | |||
| Completed planned duration | 13 (28.3%) | 9 (24.3%) | 4 (44.4%) |
| Lack of efficacy | 16 (34.7%) | 14 (37.8%) | 2 (22.2%) |
| Adverse side effects | 9 (19.6%) | 7 (18.9%) | 2 (22.2%) |
| Non-adherence | 1 (2.2%) | 1 (2.7%) | 0 (0%) |
| Still Taking | 4 (8.7%) | 3 (8.1%) | 1 (11.1%) |
| Patient requested to stop | 1 (2.2%) | 1 (2.7%) | 0 (0%) |
| Insurance Coverage Issues | 2 (4.3%) | 2 (5.4%) | 0 (0%) |
| aDisease status defined as: active disease (UPCr > 2 mg/mg, low albumin, or edema) vs. remission (UPCr ≤ 0.5 mg/mg) Abbreviations: ACEi: Angiotensin-Converting-Enzyme; BSA: Body Surface Area; IQR: Interquartile Range; IU: International Unit |
|||
At the time of this report or last patient follow-up, 41/46 (92%) patients had discontinued use of Acthar Gel. Reasons for terminating Acthar Gel therapy included: lack of clinical response (16/41, 35%; median duration of treatment of 148 days [range, 48-661]), side effects (9/46, 20%; median duration of treatment of 57 days [range, 20-806]), planned end of course (13/46, 28%; median duration of treatment of 222 days [range, 169-950]). One patient (2%) stopped therapy and one patient was discontinued due to non-adherence (2%). Two patients (4%) discontinued due to issues with insurance coverage. Four patients are still taking Acthar Gel (4/46, 9%, median duration of treatment of 990 days [range, 519-1825]). The side effects listed as reasons for treatment termination included: relapse and abdominal pain with enteritis leading to hospitalization (n = 1), fatigue (n = 1),hypertension (n = 2), weight gain and mood swings (n = 1), severe anasarca leading to hospitalization (n = 1), fluid retention/ascites (n = 3).
Treatment response to Acthar Gel Detailed descriptions of each patient’s clinical characteristics, prior and concomitant therapies at the start of Acthar Gel, duration of Acthar Gel treatment, response to Acthar Gel, and reason for stopping Acthar Gel therapy (if applicable) are provided in Table 3.
| Table 3: Detailed clinical descriptors, Acthar Gel treatment, and treatment outcomes of 46 pediatric patients with nephrotic syndrome | |||||||
| Patient | Steroid Response Classification | Disease Status at Start of Acthar® Gel | Prior NS Treatments | Concomitant Medication at Start of Acthar® Gel | Acthar® Gel duration (days) | BOR CR, PR, or NR Or Time to relapse (days) / # of relapses |
Reason for stopping Acthar® Gel |
| Minimal Change Disease | |||||||
| 1 | Resistant | Remission | CST, MP,TAC, ACEi | CST, TAC, ACEi | 57 | 49 / 1 | Adverse Side Effects |
| 2 | Responsive | Relapse | CST, MP, RTX, MMF, TAC, ACEi, CTX | CST, TAC, ARBs | 222 | PR | Completed planned course |
| 3 | Responsive | Relapse | CST, MP, TAC, ACEi | CST, TAC, ACEi, ARBs | 806 | CR | Adverse Side Effects |
| 4 | Responsive | Relapse | CST, MP, CTX | CST | 147 | NR | Not effective |
| 5 | Resistant | Relapse | CST, MP, RTX, MMF, TAC, ACEi, CTX | CST | 54 | NR | Adverse Side Effects |
| 6 | Responsive | Relapse | CST, MP, MMF, CSA | CST, CSA, MMF, ACEi | 246 | CR | Completed planned course |
| 7 | Responsive | Remission | CST, MP, MMF, CTX, CSA | CSA, MMF, ACEi | 229 | 69 / 2 | Completed planned course |
| 8 | Responsive | Relapse | CST, MMF | CST, MMF | 196 | CR | Completed planned course |
| 9 | Resistant | Relapse | CST, ACEi | ACEi | 116 | NR | Not effective |
| 10 | Responsive | Remission | CST, MP, MMF, TAC, ACEi | CST, ACEi | 519 | 141 / 2 | N/Ab |
| 11 | Responsive | Relapse | CST, CTX, CSA | CST | 88 | NR | Not effective |
| 12 | Responsive | Remission | CST, MP, RTX, CTX, CSA, TAC | TAC | 950 | 259 / 2 | Completed planned course |
| 13 | Responsive | Relapse | CST, CTX, TAC | CST ,TAC, ACEi | 267 | NR | Not effective |
| 14 | Resistant | Relapse | CST, CTX, TAC | CST ,TAC, ACEi | 365 | NR | Not effective |
| 15 | Resistant | Relapse | CST, MP, MMF, CSA, TAC, ACEi | CSA, ACEi | 237 | NR | Not effective |
| 16 | Responsive | Relapse | CST, TAC, MMF | CST, TAC | 347 | CR | Completed planned course |
| 17 | Responsive | Relapse | CST, TAC, ACEi | CST, ACEi | 148 | NR | Not effective |
| 18 | Resistant | Relapse | TAC | CST, TAC | 82 | PR | Adverse Eventf |
| Focal Segmental Glomerulosclerosis | |||||||
| 19 | Resistant | Relapse | CST, MP, RTX, MMF, TAC, ACEi | MMF, TAC, ACEi, | 1825 | CR | N/Ab |
| 20 | Responsive | Relapse | CST, TAC, ACEi | ACEi, | 203 | NR | Completed planned course |
| 21 | Resistant | Relapse | CST, MMF, TAC, ACEi | ACEi | 156 | NR | Not effective |
| 22 | Resistant | Relapse | CST, MP, MMF, ACEi, CTX, CSA | CST, MMF, CSA, ACEi | 138 | PR | Not effective |
| 23 | Resistant | Relapse | CST, MP, MMF, ACEi, CSA | MMF, ARBs, ACEi | 201 | CR | Completed planned course |
| 24 | Responsive | Relapse | CST, MMF, CSA | CST, CSA, MMF, ARBs | 20 | NR | Adverse Side Effects |
| 25 | Resistant | Remission | CST, RTX, TAC | CST, TAC, ACEi, ARBs, | 21 | --/0 | Adverse Side Effects |
| 26 | Responsive | Remission | CST, MP, RTX, MMF, TAC, CTX | MMF, TAC, ACEi | 475 | 358 / 2 | Not effective |
| 27 | Responsive | Relapse | CST, MP, RTX, MMF, TAC, ACEi, CTX, CSA | CST, RTX | 491 | CR | Completed planned course |
| 28 | Resistant | Relapse | CST, MP, TAC, ACEi, CSA | CST | 88 | NR | Not effective |
| 29 | Responsive | Remission | CST, RTX, MMF, CSA, TAC, | CST | 53 | 31 / 1 | Not effective |
| 30 | Resistant | Relapse | CST, MP, RTX, MMF, CSA, TAC, ACEi | CST, ACEi | 661 | PR | Not effective |
| 31 | Responsive | Remission | CST, MP, RTX, CTX, TAC, ACEi | TAC, ACEi | 198 | --/0 | Completed planned coursee |
| 31 | Relapse | Rtx, ACEi | 226 | NR | Adverse Side Effectse | ||
| 32 | Resistant | Relapse | CST, CTX, CSA, TAC, ACEi | TAC | 153 | PR | Issues with insurance coverage |
| 33 | Resistant | Relapse | CST, MP, RTX, MMF, TAC | MMF, TAC | 493 | NR | Adverse Side Effects |
| 34 | Responsive | Relapse | TAC | CST, TAC | 71 | NR | Not effective |
| IgA Nephropathy | |||||||
| 35 | Resistant | Relapse | CST, MMF, ACEi | CST, ACEi | 169 | PR | Completed planned course |
| 36 | Resistant | Relapse | CST, MMF, ACEi, AZA | ARBs | 282 | CR | Adverse Side Effects |
| Membranoproliferative Glomerulonephritis | |||||||
| 37 | N/A1 | Remission | MMF | ACEi | 826 | --/0 | Completed planned course |
| 38 | Resistant | Relapse | CST, RTX, MMF, CSA, TAC, ACEi | -- | 179 | NR | Issues with insurance coverage |
| Membranous Nephropathy | |||||||
| 39 | Resistant | Relapse | CST, ACEi | ACEi | 511 | CR | Patient Requested to Stop |
| 40 | N/A1 | Relapse | ACEi | ACEi | 178 | NR | Completed planned course |
| Systemic Lupus Erythematosus | |||||||
| 41 | Resistant | Relapse | CST, RTX, MMF, TAC, ACEi | CST, TAC, MMF, ARBs, ACEi | 525 | PR | N/Ab |
| 42 | Resistant | Relapse | CST, MMF, ACEi | CST, MMF, ACEi | 68 | PR | Non-adherence |
| Post-transplant Recurrent FSGSd | |||||||
| 43 | Resistant | Relapse | CST, MP, MMF, TAC, RTX, PP | CST, MMF, TAC | 1455 | CR | N/Ab |
| 44 | Resistant | Relapse | CST, RTX, TAC, MMF, PLEX, AZA | CST, TAC, MMF, ACEi, | 23 | NR | Adverse Eventg |
| 45 | Resistant | Relapse | CST, RTX, CSA, ACEi, AZA | CST, CSA, AZA | 192 | NR | Not effective |
| No Biopsy | |||||||
| 46 | Responsive | Relapse | CST | -- | 48 | NR | Not effective |
|
|||||||
Among the 9 patients who were in disease remission at the start of Acthar Gel treatment, 3 remained in remission while on therapy (Patients 25, 31, and 37), with duration of treatment of 2 36, 198 and 826 days. Of note, Patient 1 stopped Acthar Gel treatment after 57 days due to adverse effects. Six patients relapsed during Acthar Gel therapy (Patients 1, 7, 10, 12, 26, and 29) with a median time-to-relapse was 352 days (IQR 100 – 508 days). Of note, Patient 7 had 2 relapses in 229 days Patient 10 had 2 relapses in 519 days, and Patient 12 had 3 relapses in 950 days which would be classified as infrequent disease relapses [2]. The total oral corticosteroid exposure in the 6 months pre and post Acthar Gel treatment is presented in Table 4. Patient 1, 10, and 26 had a large reduction in corticosteroid exposure in the 6 months after Acthar Gel treatment as compared to the previous 6 months.
| Table 4: Acthar Gel treatment response among nine pediatric nephrotic syndrome patients who were in disease remission at start of therapy. | ||||
| Patient | CST exposure in Prior 6m (mg) | Duration of Acthar Gel Treatment (d) | # of Relapses on Acthar Gel | CST exposure 6m post Acthar Gel (mg) |
| 1 | 3,609 | 57 | 1 | 1,836 |
| 7 | 900 | 229 | 2 | 1,350 |
| 10 | 5,200 | 519 | 2 | 2,260 |
| 12 | 0 | 950 | 3 | 0 |
| 25 | 0 | 36 | 0 | 0 |
| 26 | 6,540 | 475 | 2 | 0 |
| 29 | 3,810 | 53 | 1 | 4,940 |
| 31 | 0 | 198 | 0 | 0 |
| 37 | 10 | 826 | 0 | 0 |
| Abbreviations: CST: Oral Corticosteroids; D: Days; M: Months | ||||
Predicators of Acthar Gel response
Patients initially showing active disease with subsequent partial or complete remission while on Acthar Gel treatment were considered responders to Acthar Gel. Univariable analysis examining the associations between patient characteristics and Acthar Gel response is presented in
Table 5. Of note, all patients demonstrating an Acthar Gel response had concomitant use of immunosuppressive therapy at the start of Acthar Gel but failed to achieve statistical significance (p = 0.08). Concomitant Mycophenolate Mofetil at the start of Acthar Gel was more common in responders compared to non-responders (44% vs. 16%, p = 0.06).
| Table 5: Univariable analysis examining associations between patient characteristics and Acthar Gel responsea among pediatric patients with active nephrotic syndrome at the start of Acthar Gel treatment. | |||
| Characteristic | Non-Responder N = 19 |
Responder N = 18 |
p |
| Age at Diagnosis--yr, median (IQR) | 3.5 (2.0 – 10.3) | 5.9 (4.4 – 10.7) | 0.11 |
| Age at Acthar Gel Start--yr, median (IQR) | 12.5 (8.5 – 18.4) | 12.3 (9.0 – 16.6) | 0.89 |
| Female Sex—n (%) | 6 (31.6%) | 9 (50.0%) | 0.25 |
| Non-Hispanic Ethnicity—n (%) | 12 (63.2%) | 13 (72.2%) | 0.37 |
| Caucasian Race—n (%) | 13 (68.4%) | 10 (55.6%) | 0.42 |
| Hypertension Diagnosis—n (%) | 9 (47.4%) | 5 (27.8%) | 0.22 |
| Diagnosis—n (%) | 0.47 | ||
| MCD | 8 (42.1%) | 6 (33.3%) | |
| FSGS | 6 (31.6%) | 6 (33.3%) | |
| IgAN | 0 (0%) | 2 (11.1%) | |
| MPGN | 1 (5.3%) | 0 (0%) | |
| MN | 1 (5.3%) | 1 (5.6%) | |
| SLE | 0 (0%) | 2 (11.1%) | |
| No biopsy | 1 (5.3%) | 0 (0%) | |
| Post-transplant recurrent FSGS | 2 (10.5%) | 1 (5.6%) | |
| Steroid Classification | 0.49 | ||
| Steroid Responsive | 8 (42.1%) | 6 (33.3%) | |
| Steroid Resistant | 10 (52.6%) | 12 (66.7%) | |
| No Previous Steroid Therapy | 1 (5.3%) | 0 (0%) | |
| Number of Previous IST--median (IQR) | 2 (2 – 4) | 3 (2 – 5) | 0.23 |
| UPCr start of Acthar Gel --median (IQR) (n = 28) | 6.1 (1.8 – 9.7) | 4.1 (2.4 – 5.6) | 0.33 |
| Concomitant Medications at Start of Acthar® Gel —n (%) | |||
| Oral Corticosteroids | 11 (57.9%) | 13 (72.2%) | 0.36 |
| Mycophenolate Mofetil | 3 (15.8%) | 8 (44.4%) | 0.06 |
| Cyclosporine | 3 (15.7%) | 2 (11.1%) | 0.68 |
| Tacrolimus | 5 (26.3%) | 8 (44.4%) | 0.25 |
| ACEi/ARBs | 10 (52.6%) | 12 (66.7%) | 0.38 |
| Continued on concomitant medications | 16 (84.2%) | 18 (100%) | 0.08 |
| aResponse to Acthar® Gel for patients with active disease at the start of Acthar® Gel was classified as: Complete Remission (CR) - serum albumin ≥ 3.5 g/dL, UPCr < 0.5 mg/mg, and no edema; partial Remission (PR) - an increase in serum albumin > 0.5 g/dL and decrease in UPCr by > 50%; and NR - worsening or no improvements in serum albumin > 0.5 g/dL or UPCr > 50%. Abbreviations: ACEi: Angiotensin-Converting-Enzyme inhibitors; FSGS: Focal Segmental Glomerulosclerosis; IgAN: Iga Nephropathy; IQR: Interquartile Range; IST: Immunosuppressive Therapy; Yr: Year; MCD: Minimal Change Disease; MN: Membranous Nephropathy; SLE: Systemic Lupus Erythematosus; UPCr: Urine Protein to Creatinine Ratio. | |||
Our registry represents the largest reported cohort of pediatric patients treated with Acthar® Gel for nephrotic syndrome in the United States. Our data suggests that in recent years U.S. pediatric nephrologists used Acthar Gel to treat children with difficult-to-treat nephrotic syndrome with varied underlying histologic diagnoses. The patients in the registry in general had long-standing disease with high steroid-resistance and exposure to many second-line immunosuppressive agents prior to Acthar Gel use. Responses to Acthar Gel treatment were variable, with approximately half of the patients with active disease displaying improvements in serum albumin and proteinuria or decreased corticosteroid exposure. Responders received a variety of immunosuppressive medications and antiproteinuric agents concomitantly with Acthar Gel treatment.
Historically, ACTH was used in the 1950s in treatment-naïve pediatric patients as a single agent before oral prednisone became available. In contrast, the current cohort of patients represent difficult-to-treat cases far from initial diagnosis with 44 out of 46 patients treated with immunosuppressive agents other than corticosteroids. In a meta-analysis of studies conducted between 1945 to 1965 of ACTH use after initial diagnosis, proteinuric response was shown in 71% of patients and sustained up to 4.7 years following treatment [4]. Not surprisingly, the response to Acthar Gel in our cohort is significantly worse likely secondary to selection of a patient population that was more resistant to corticosteroids. The use of the new Acthar Gel formulation and the use of Acthar Gel in patients who have failed other immunosuppressive agents are similar to recent adult case series. Hogan et al. reported 24 patients with FSGS treated with Acthar Gel. Sixty percent of the patients were steroid-resistant and 92% were exposed to other immunosuppressive agents. Thirty percent of patients in that cohort achieved complete or partial remission [13]. In the largest adult case series, Madan, et al. reported 44 adult patients with varied diagnoses, with 68% of the patients receiving prior immunosuppressive agents. Sixty-two percent showed proteinuria reduction of ≥ 50% [9]. It is important to point out that direct comparisons to this case series is difficult, as the adult series contained a large number of membranous nephropathy patients and a few diabetic nephropathy and fibrillary glomerulonephritis patients. In addition, we included 2 patients with post-transplant recurrent FSGS.
Though our findings suggest potential beneficial effects of Acthar Gel for treatment-resistant nephrotic syndrome, due to the observational nature of this study, it is challenging to reach conclusions regarding the efficacy of Acthar Gel for the treatment of nephrotic syndrome. Our interpretation is further complicated by the highly variable dosing and treatment duration prescribed among patients in the cohort. In particular, the duration of Acthar Gel treatment is significantly shorter (p < 0.01) among patients who failed to achieve either partial or complete response, with 7/19 (37%) patients terminating treatment in less than 90 days. The prescribed Acthar Gel duration in our cohort was thus highly dependent on early response within the first 3 months of treatment, i.e. the drug was more likely to be continued in patients who demonstrated early response. This may have negatively skewed the interpretation of the efficacy of the drug, as one pilot study in adult patients with MN found a trend towards increased efficacy in patients with higher cumulative exposure of Acthar Gel [14]. Importantly, the majority of patients in our cohort continued other immunosuppressants in addition to Acthar Gel, making it difficult to ascribe the response to any one treatment. In our unadjusted exploratory analysis, only the use of mycophenolate at the start of Acthar Gel and continued use of other immunosuppressants along with Acthar Gel were more common among patients with response to treatment but failed to reach statistical significance. This result highlights the issue of confounding when concomitant agents are given.
In a recent randomized clinical trial comparing Acthar Gel treatment to no maintenance therapy for the treatment of steroid-sensitive childhood nephrotic syndrome (ATLANTIS, Clinicaltrials.gov identifier: NCT02132195), Acthar Gel was found to be ineffective at preventing disease relapses [11]. The enrollment period of the current cohort occurred before the publication of this trial, and we found that more than half of the cohort were steroid-resistant as opposed to steroid-dependent or frequently relapsing. In addition, the majority of patients in our cohort (80%) began Acthar Gel with active disease, as opposed to a remission state in the ATLANTIS trial. We thus utilized different outcomes to examine the efficacy of Acthar Gel - reduction of proteinuria and improvements in serum albumin and edema among those with active disease at the start of treatment in addition to time-to-relapse and corticosteroid exposure among those in a remission state at the start of Acthar Gel. A comparison of the results of this registry with the controlled ATLANTIS trial is not possible, not least because of the controlled versus observational designs, but also due to differences in the patient population and outcomes examined. There were too few patients in disease remission (n = 9) at the start of Acthar Gel treatment to draw conclusions of the drug in preventing relapses. We believe that the results of this registry suggest that a prospective trial using Acthar Gel in treatment-resistant childhood nephrotic syndrome is needed to help guide pediatric nephrologists in the treatment of this difficult group, with a controlled treatment duration > 3 months and examining outcomes relevant to this group: reduction in UPCr and improvements in serum albumin and edema.
Based on our registry data we conclude that in current U.S. practice, Acthar Gel was given for highly variable diagnoses in pediatric nephrotic syndrome, generally in patients with severe and prolonged disease. Prescribed drug duration and usage with other immunosuppressants were highly variable, making it difficult to interpret response to treatment. Given that Acthar Gel is given via injection, which may be difficult for many children and their caregivers, and the extraordinary expense of treatment [15], more evidence is clearly needed. We propose a randomized prospective trial with steroids as a control arm, or a prospective study in treatment-resistant childhood nephrotic syndrome with standardized treatment duration of Acthar Gel to guide clinicians in the treatment of this difficult disease.
Compliance with ethical standards
Ethical approval: The study was approved by the institutional review board (IRB) at each participating center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent: Written parental consent and minor assent were obtained in accordance with local IRB guidelines.
Funding: This study was an investigator-initiated study supported by Mallinckrodt Pharmaceuticals, makers of Acthar® Gel. The sponsors had no role in the design of the study, evaluation of the results or writing of the manuscript.
Conflict of interest: CM, MKF, TL, and MRB receive additional research support from Mallinckrodt Pharmac-euticals. MKF received research support from Reata Pharmaceuticals. KL received speaking honoraria from Mallinckrodt Pharmaceuticals.
We wish to acknowledge the Steering Committee, members, and staff of the North American Pediatric Trials and Collaborative Studies (NAPRTCS) and the Pediatric Nephrology Research Consortium (PNRC) who facilitated and participated in this study. We would like to thank the members of the Scientific Review Committee for their time and thoughtful evaluation of the study.
- Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome--current and future therapies. Nat Rev Nephrol. 2012; 8: 445-458. PubMed: https://pubmed.ncbi.nlm.nih.gov/22688744/
- Lombel RM, Gipson DS, Hodson EM, Kidney Disease: Improving Global Outcomes: Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol. 2013; 28: 415-426. PubMed: https://pubmed.ncbi.nlm.nih.gov/23052651/
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003; 362: 629-639. PubMed: https://pubmed.ncbi.nlm.nih.gov/12944064/
- Lieberman KV, Pavlova-Wolf A. Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: a systematic review of early clinical studies with contemporary relevance. J Nephrol. 2017; 30: 35-44. PubMed: https://pubmed.ncbi.nlm.nih.gov/27084801/
- Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2011; 8: 122-128. PubMed: https://pubmed.ncbi.nlm.nih.gov/22143333/
- Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004; 19: 1305-1307. PubMed: https://pubmed.ncbi.nlm.nih.gov/15102969/
- Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999; 56: 1534-1543. PubMed: https://pubmed.ncbi.nlm.nih.gov/10504505/
- Bomback AS, Tumlin JA, Baranski J, Bourdeau JE, Besarab A, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011; 5: 147-153. PubMed: https://pubmed.ncbi.nlm.nih.gov/21448451/
- Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, et al. Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016; 17: 37. PubMed: https://pubmed.ncbi.nlm.nih.gov/27036111/
- Mallinckrodt Pharmaceuticals. Acthar Gel. 2019.
- Wang CS, Travers C, McCracken C, Leong T, Gbadegesin R, et al. Adrenocorticotropic Hormone for Childhood Nephrotic Syndrome: The ATLANTIS Randomized Trial. Clin J Am Soc Nephrol. 2018; 13: 1859-1865. PubMed: https://pubmed.ncbi.nlm.nih.gov/30442868/
- Wang CS, Greenbaum LA. Nephrotic Syndrome. Pediatr Clin North Am. 2019; 66: 73-85. PubMed: https://pubmed.ncbi.nlm.nih.gov/30454752/
- Hogan J, Bomback AS, Mehta K, Canetta PA, Rao MK, et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013; 8: 2072-2081. PubMed: https://pubmed.ncbi.nlm.nih.gov/24009220/
- Hladunewich MA, Cattran D, Beck LH, Odutayo A, Sethi S, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar(R) Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014; 29: 1570-1577. PubMed: https://pubmed.ncbi.nlm.nih.gov/24714414/
- Hartung DM, Johnston K, Deodhar A, Bourdette DN, Cohen DM. Repository Corticotropin Versus Glucocorticoid for Nephrotic Syndrome: When Will We See the Evidence? Am J Kidney Dis. 2019; 74: 256-262. PubMed: https://pubmed.ncbi.nlm.nih.gov/30765104/
- Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009; 20: 629-637. PubMed: https://pubmed.ncbi.nlm.nih.gov/19158356/