More Information
Submitted: January 12, 2024 | Approved: January 27, 2024 | Published: January 29, 2024
How to cite this article: Giuseppe P, Fortuna P, Chiara GM, Del Prete C, Caterina L, et al. Cognitive Impairment in Renal Replacement Therapy: Comparison between Methods. J Clini Nephrol. 2024; 8: 001-007.
DOI: 10.29328/journal.jcn.1001119
Copyright License: © 2024 Giuseppe P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Peritoneal dialysis; Cognitive impairment; Cognitive reserve index questionnaire; Chronic kidney disease
Cognitive Impairment in Renal Replacement Therapy: Comparison between Methods
Paribello Giuseppe1* , Papa Fortuna1, Ganzerli Maria Chiara1, Del Prete Chiara1, Lanzuise Caterina1, Capuano Ivana2, Pacella Daniela3, Sannino Giuseppina1, Rompianesi Gianluca4, Pisani Antonio1 and Riccio Eleonora1
, Papa Fortuna1, Ganzerli Maria Chiara1, Del Prete Chiara1, Lanzuise Caterina1, Capuano Ivana2, Pacella Daniela3, Sannino Giuseppina1, Rompianesi Gianluca4, Pisani Antonio1 and Riccio Eleonora1
1Federico II University Hospital, Nefrology, Italy
2San Giuliano Hospital, Nephrology, Italy
3Federico II University Hospital, Department of Public Health, Italy
4Federico II, University Hospital, Department of Clinical Medicine and Sugery, Italy
*Address for Correspondence: Paribello Giuseppe, Federico II University Hospital, Nefrology, Italy, Email: [email protected]
Cognitive impairment (CI) can be defined as a clinical syndrome characterized by a decline in at least two of several domains of cognitive function. Chronic kidney disease (CKD) is an independent risk factor for cognitive decline, and the prevalence in patients with end-stage renal disease is estimated at 50% - 80%. However, it appears that CI in patients on renal replacement therapy (RRT) may be underdiagnosed. In this cross-sectional study, 33 patients on Peritoneal Dialysis from the AOU Federico II were recruited, and matched by sex, age, and dialysis age to 33 patients on Hemodialysis and 33 controls belonging to healthy volunteers. The total 66 patients and their 33 controls were assessed for cognitive function using the Cognitive Reserve Index Questionnaire (CRIq) test. Between PD and HD patients, a statistically significant difference emerged in all subscores and in the total CRI. Between PD patients and controls, a statistically significant difference emerged in education, CRI- CRI-leisure time, and the total CRI. Therefore, CI may occur in patients undergoing PD earlier and with a greater frequency than in the general population, but with a lower incidence than in patients on HD. These considerations should be communicated to patients when they are educated about different replacement methods.
Cognitive Impairment (CI) can be defined as a clinical syndrome characterized by a decline in at least two of several domains of cognitive function.
The Alzheimer’s Association believes there is sufficient evidence to support the link between several modifiable risk factors and a reduced risk for cognitive decline [1]. Among the risk factors are:
- Cardiovascular risk factors: Diabetes; Mid-life obesity; Hyperlipidemia (elevated cholesterol)
- Lifestyle risk factors: Current smoking; Alcohol
- Other risk factors: Traumatic brain injury; Depression: Sleep disturbances
Instead, the factors that decrease the risk of CI are Physical activity, Mediterranean diet, Cognitive training, Social engagement, Years of formal education [1], Chronic Kidney Disease (CKD) is an independent risk factor for cognitive decline, and the prevalence in patients with end-stage renal disease is estimated at 50% - 80%. Problems with cognitive functioning have also been observed in patients undergoing renal replacement therapy (RRT), both Peritoneal Dialysis (PD) and Hemodialysis (HD), even in non-elderly patients. However, it appears that CI in patients on RRT may be underdiagnosed.
Cognitive impairment in peritoneal dialysis
The pathophysiology of cognitive impairment in peritoneal dialysis patients is undefined, but likely has a large vascular ischemic component combined with neurodegenerative pathology, exacerbated by chronic inflammation in hemodialysis patients, the dialysis process results in large acute intravascular volume loss and fluid shifts leading to cerebral edema, decreased cerebral perfusion, and cerebral ischemia, all of which may contribute to cognitive impairment. However, peritoneal dialysis does not involve the same extent of acute fluid and electrolyte shifts; thus, the high prevalence of stroke and elevated levels of inflammation, uremia, and cardiovascular risk factors among peritoneal dialysis patients may be the strongest contributors to cognitive impairment [2].
Cognitive impairment and cardiovascular system: Most patients with End State Renal Disease (ESRD) have diffuse vasculopathy with secondary elevated rates of cardiovascular events [2-4]. Although stroke doubled the risk of severe cognitive impairment in the hemodialysis cohort, it was not a risk factor in the peritoneal dialysis cohort. This may be due to the low occurrence and underreporting of stroke in this cohort and to low statistical power [2,5].
Cognitive impairment and nervous system: In previous studies, on brain magnetic resonance imaging (MRI), it has been found that white matter disease (leukoaraiosis) was present in 68% of 57 peritoneal dialysis patients [6]. White matter disease found on MRI corresponds to vascular degenerative morphology consistent with chronic hypoxia and vascular hypoperfusion on autopsy. These pathologic changes include hyalinosis of blood vessels, demyelination of neurons, endothelial and microglial activation, and increased levels of molecular markers of hypoxia such as hypoxia-inducible factors and matrix metalloproteinase [2,7]. White matter disease is associated with an increased risk of stroke, disability, cognitive impairment, and decline [2,8,9].
Cognitive impairment and metabolism: Daily prolonged exposure to the high glucose load in the peritoneal dialysis dialysate is associated with weight gain (fat deposition), hyperleptinemia, and dyslipidemia, which may lead to increased risk of cardiovascular disease including micro-and macrocerebrovascular disease [2,10]. Mid-abdominal obesity and metabolic syndrome, prevalent in peritoneal dialysis patients, have also been identified as risk factors for dementia in nondialysis patients [2,11-14].
Cognitive impairment and hemodialysis
Significant changes in the circulatory system during hemodialysis cause impairment of tissue blood supply, including the central nervous system. The reason for this is the loss of water, both in the ultrafiltration process and from migration to tissues from blood vessels. This leads to a decrease in blood volume, an increase in its density and viscosity, and a resistance increase [7].
Cognitive impairment and systemic cardiovascular risk factors: Many studies have found that Pulse Wave Velocity (PWV) and Ankle-Brachial Index (ABI) are important factors associated with cognitive impairment in dialysis patients. These parameters have been validated as tools for assessing arterial health as measured by arterial stiffness. The first scientific study found that having a high PWV or a low ABI is associated with poor cognitive function in HD patients [15]. Other studies support the link between PWV and cognitive impairment in hemodialysis patients [16]. Orthostatic pressure reduction is another cardiovascular factor linked to cognitive performance. According to the findings of the study, an excessive reduction in orthostatic pressure in HD patients causes memory impairment [17]. Another factor to consider is left ventricular function. CKD increases the risk of developing left ventricular hypertrophy at a young age. In the early stages of HD, 70% - 80% of patients have left ventricular hypertrophy [13,17-19]. Furthermore, chronic hemodialysis reduces cerebral blood flow, which may exacerbate the effects of low left ventricular function (LVEF) [20]. According to research, a mildly reduced LVEF correlates with cognitive impairment [21].
Cognitive impairment and nervous system: It has been proposed that hemodialysis causes brain damage associated with recurrent hemodynamic changes, specifically a decrease in cerebral intradialytic perfusion. Dialysis factors such as ultrafiltration volume or intradialytic hypotension could be one cause of this phenomenon [21-22].
Cognitive impairment and markers related to the inflammatory process and cell damage: Inflammatory cytokines play an important role in the pathogenesis of hemodialysis-related side effects associated with brain diseases and it has been found that IL-6 and TNF- levels increased significantly in HD patients [23-26].
The next marker under consideration is fibroblast growth factor-23 (FGF-23), whose level is elevated in HD patients and is linked to left ventricular hypertrophy and increased mortality [27,28]. In another study, elevated levels of FGF23 were linked to memory deterioration as measured by composite memory scores. This suggests that in HD patients, this marker may contribute to CI [2,29].
The study aimed to determine the frequency of CI in patients undergoing PD and compare them with patients undergoing HD and a control group.
In this cross-sectional study, 33 patients on Peritoneal Dialysis from the AOU Federico II were recruited, and matched by sex, age, and dialysis age to 33 patients on Hemodialysis and 33 controls belonging to healthy volunteers.
This study was conducted in accordance with the standards of the International Conference on Harmonization-Good Clinical Practice and the Declaration of Helsinki.
The study was approved by the Institutional Ethical Committee of Federico II Hospital (Protocol n. 85/2023)
No sponsor was involved in the study design, recruitment, and data analysis phases.
Patients were included in the study only after signing the Informed Consent. This consent could be withdrawn at any time during the study and for any reason and had no effect on the clinical management of the participants.
Socio-demographic data (sex, age, smoking, weight, height, dialysis age) and details of the clinical and pharmacological history were collected at the beginning of the observation for each individual patient.
The total 66 patients and their 33 controls were assessed for cognitive function using the Cognitive Reserve Index Questionnaire (CRIq) test.
The CRIq includes demographic data and items grouped into three main sections, each of which returns a subscore:
- CRI-Education (number of years of school attended);
- CRI-Working Activity (type of profession and duration of employment);
- CRI-Leisure Time (extra-work activities at various weekly, monthly, annual, and fixed frequencies).
The administration of the CRIq in a clinical setting requires that the interviewee has sufficiently intact cognitive functions to be able to make an objective analysis of their entire life. In the case of patients with cognitive deficits in memory or attention or with brain deterioration, it is possible that the questions are asked of a family member.
The final score of the CRI questionnaire is the average of the three indices transposed onto a scale with a mean of 100 and standard deviation of 15, as well as the sub-indexes of the three sections. In this way, the CRI score of each subject is estimated net of his age. The CRIq scores are summarized in Table 1.
| Table 1: CRIq scores. | ||||
| Low | Medium-low | Medium | Medium-high | High |
| ≤ 70 | 70:84 | 85:114 | 115:130 | ≥130 |
Patients with psychiatric or neurodegenerative disorders and delirium were excluded.
According to the literature, we also evaluated possible risk factors for cognitive impairment: the presence of Diabetes, the presence of Obesity, Presence of hyperlipidemia, and current smoking [1].
Inclusion criteria
The inclusion criteria for this study were:
- Adult subjects (> 18 years)
- Start of renal replacement therapy at least 6 months ago
- Signature of the Informed Consent
- Absence of previous psychiatric or neurodegenerative disorders and delirium
Statistical analysis
Results are expressed as the mean ± SD and as absolute frequency (percentage).
The normality of the variable distributions was assessed using the Shapiro-Wilk test.
The difference between means for two different groups (patients in peritoneal dialysis vs patients in hemodialysis and patients on peritoneal dialysis versus controls) was determined using the Wilcoxon-Mann-Whitney test.
The difference in prevalence of risk factors for CI in the three groups was analyzed using Fisher’s exact test. For all analyses, p - value < 0.05 was considered significant.
These analyses were performed with SPSS statistical software version 20.
Table 2 shows the main socio-demographic characteristics of the patients and the control group. Per protocol, the characteristics reported are comparable in the 3 groups.
| Table 2:Socio-demographic characteristics of the patients and the control group. | ||||
| Peritoneal Dialysis | Hemodialysis | Controls | ||
| Total | 33 | 33 | 33 | |
| Male/Female | 18 (55%) | 19 (58%) | 17 (52%) | |
| Female | 15 (45%) | 14 (42%) | 16 (48%) | |
| Middle Age (years) | 61±5,2 | 65,2±3,4 | 63±3,6 | |
| Education Level | Primary school diploma | 5 | 7 | 6 |
| Middle school diploma | 8 | 12 | 7 | |
| Higher diploma | 15 | 11 | 14 | |
| Degree | 5 | 3 | 6 | |
| Dialysis Age (months) | 18,4 ± 5,4 | 21 ± 3,7 | N/A | |
| Peritoneal Dialysis Method (CAPD) | 18 (55%) | N/A | N/A | |
| Peritoneal Dialysis Method (APD) | 15 (45%) | N/A | N/A | |
| Residual Diuresis (ml) | 1500 ± 260 | 335 ± 120 | 1750 ± 340 | |
| Diabetes | 3 (9%) | 6 (18%) | 2 (6%) | |
| Obesity | 7 (21%) | 7 (21%) | 7 (21%) | |
| Hyperlipidemia | 28 (85%) | 29 (88%) | 21 (64%) | |
| Smoke | 8 (24%) | 11(33%) | 7 (21%) | |
The difference between residual diuresis was statistically significant between PD patients and HD patients (p < 0.05) and between HD patients and controls (p < 0.05) but was not significant between PD patients and controls.
Table 3 shows the values of CRIq, CRI-Education, CRI-Working Activity, and CRI-Leisure Time for patients in peritoneal dialysis and patients in hemodialysis.
| Table 3:CRI scores between PD and HD patients. | |||
| Peritoneal Dialysis | Hemodialysis | p | |
| CRI- Education | 102,9 ± 17,5 | 87,6 ± 20,6 | p < 0,05 |
| CRI- Working Activity | 95,2 ± 16,6 | 89 ± 23,5 | p < 0,05 |
| CRI- Leisure Time | 101 ± 20,5 | 77,6 ± 20 | p < 0,05 |
| CRI-Index | 99,6 ± 20,4 | 85,3 ± 20,1 | p < 0,05 |
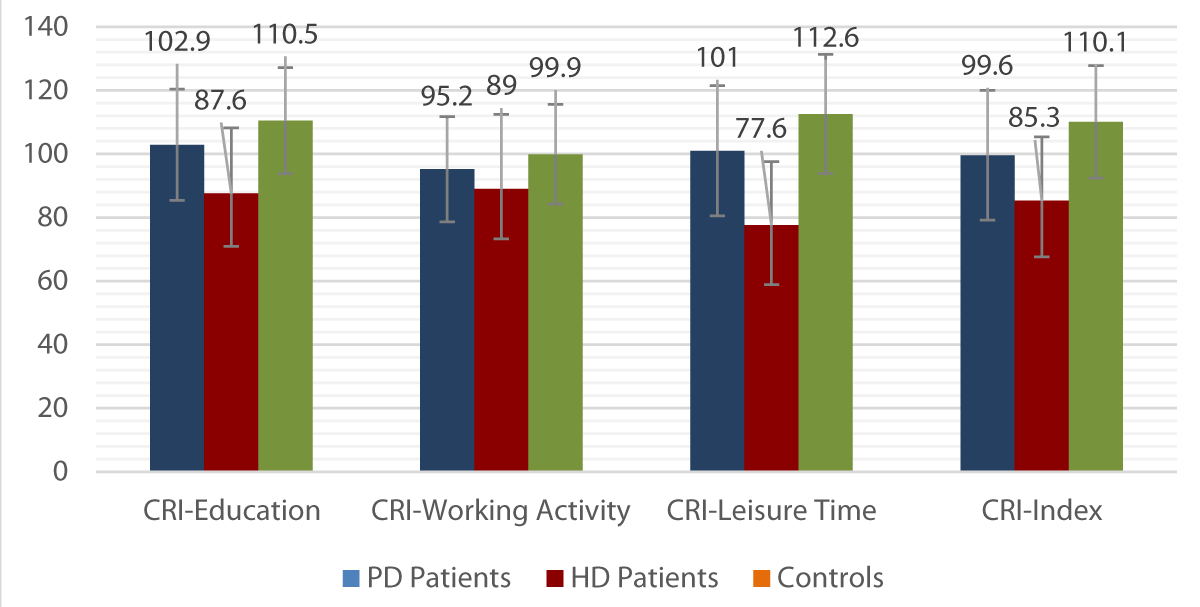
When comparing the mean scores between the three sections of the CRIq between PD and HD patients, a statistically significant difference emerged (p < 0.05) in all subscores and in the total CRI (Table 3).
Table 4 shows the values of CRIq, CRI-Education, CRI-Working Activity, and CRI-Leisure Time for patients in peritoneal dialysis and controls.
| Table 4:CRI scores between PD patients and controls. | |||
| Peritoneal Dialysis | Controls | p | |
| CRI- Education | 102,9 ± 17,5 | 110,5 ± 16,7 | p < 0,05 |
| CRI- Working Activity | 95,2 ± 16,6 | 99,9 ± 15,7 | ns |
| CRI- Leisure Time | 101 ± 20,5 | 112,6 ± 18,7 | p < 0,05 |
| CRI-Index | 99,6 ± 20,4 | 110,1 ± 17,7 | p < 0,05 |
When comparing the mean scores between the three sections of the CRIq between PD patients and controls, a statistically significant difference emerged (p < 0.05) in the CRI-Education, CRI-Leisure Time, and the total CRI, but not in CRI-Working Activity (Table 4).
The means and relative SDs of the CRIq scores in the three groups under study are illustrated in Graph 1.
Graph 1: Means and SDs of the CRIq scores in the three groups.
In PD patients, the prevalence of a medium-low CRIq level (CRI < 84), compatible with a CI, was 27% and was significantly lower than in the group of patients undergoing HD (58%) (p < 0.05), but higher than controls (6%) (p < 0.05).
No statistically significant differences were found in the prevalence of risk factors for IC in the three groups.
While it is sufficiently well documented that ESRD has been linked with a change in cognitive function and that cognitive impairment is associated with the severity of CKD [30], little is known about the influence of different dialysis modalities on cognitive function. Previous investigations of cognitive function among ESRD patients demonstrated that PD patients had consistently better cognitive function than HD patients [31-33].
Wolcott, et al. [31] reported that PD patients had consistently more efficient cognitive function than HD patients. In a study with HD patients, a higher mini-mental state exam score was associated with higher serum albumin level, protein catabolic rate and interdialysis weight gain [34].
Buoncristiani, et al. [32] concluded that the most likely reason for the better preservation of the cognitive function in CAPD patients could be due to the continuity of action, better anemia correction, more removal of the middle molecules, or lower levels of parathyroid hormone, which in some studies on HD patients have been correlated with alteration of cognitive function [32].
In 2005 Sithinamsuwan, et al. undertook a cross-sectional study on 90 ESRD subjects (60 on HD and 30 on PD) to evaluate CD using the DSM-IV criteria [35]. They found no significant difference regarding the prevalence of dementia and depression between the HD and PD groups [35].
Likewise, Lai et al. in Italy assessed the neurological, psychological, and cognitive imbalance in subjects with CKD/ ESRD on conservative and RRT, respectively [36]. They included 74 subjects, 22 of whom were clinically stable and conservatively managed CKD subjects, 15 were HD, 16 were PD, and 21 were kidney transplant patients, while 25 healthy controls were also added, matched for age and sex. The authors relied on clinical, laboratory, and non-invasive instrumental examinations (Electroencephalogram, EEG) and cognitive-psychological tests for the early identification of CI.
Mirroring Sithinamsuwan et al.’s results, these authors found no significant difference in CI between PD and HD subjects [35-36].
Taking into consideration the several limitations of the aforementioned studies might explain our disagreement with their results. The cross-sectional nature of all three studies, racial disparities, the short follow-up period, the discrepancy in tools to assess CD, the difference in dialysis duration, and the unclear baseline cognitive function in the examined groups all limit the interpretation of the results.
Moving forward, Lambert et al. undertook another large cross-sectional study in Australia including 154 subjects with renal dysfunction (23 with GFR < 30 not on dialysis, 54 HD, 25 PD, and 52 transplant subjects) [37].
The baseline cognitive functions were similar amongst the groups. The authors showed relative CD in all four groups, with the HD group having the highest prevalence. However, CD was similar in the adequately dialyzed subjects, with a relatively higher incidence in PD patients compared to results demonstrated in other studies.
Furthermore, Neumann, et al. assessed cognitive changes in a German cohort of 271 ESRD subjects (96 on HD and 101 on PD) using two validated neurocognitive tests, the Trail Making Test-B and the d2-Revision-Test to examine executive function and attention, respectively. Additionally, they applied the self-reported Kidney Disease Quality of Life Short Form Cognitive Function-subscale as well [38]. Although both modalities have shown improvements in cognitive function over a 1-year period, PD, as compared to HD, was associated with superior cognitive function outcomes at both baseline and follow-up. The largest limitation of this study was the better baseline cognitive function in PD subjects.
Taking the two prospective studies at hand, lyasere, et al. assessed the influence of different dialysis modalities on cognitive function decline in CKD and ESRD subjects [39].
The authors used the MoCA tool to evaluate CD in 102 subjects (41 HD, 25 PD, and 36 CKD). The study showed that cognition declines faster in dialysis subjects compared to CKD subjects and in HD subjects compared to PD subjects. Comparably, Neumann et al. also conducted a larger prospective study to assess cognitive function in 767 subjects (527 HD and 240 PD) using the Trail Making Test-B, the German d2-Revision Test, as well as the Kidney Disease Quality of Life Short Form cognition subscale [40]. Again, the authors concluded that HD subjects carry an additive risk of CI compared to PD subjects. Nevertheless, the prospective nature of both studies, the longer follow-up period (2 years) in lyasere, et al.’s study, and the larger sample size in Neumann et al.’s study provided further validity to the authors’ results [39,40].
A recent meta-analysis included 15 cohort and cross-sectional studies and compared qualitative and quantitative cognitive functions in peritoneal and hemodialysis subjects [41]. These authors concluded that PD subjects had better cognitive functions and a lower risk of cognitive decline compared to HD subjects.
Overall, our data confirm the findings of all prospective studies and most available cross-sectional studies. Indeed, after excluding the possible influence of other risk factors, we found a faster cognitive impairment in dialysis patients compared to the control population and a faster cognitive impairment in hemodialysis patients compared to peritoneal dialysis patients. The reasons behind this observation are different: subjects on PD are subjected to daily treatment compared to the majority of subjects on HD treated only three times a week. The rapid movement of molecules in subjects on HD certainly has an impact on cognitive decline, as evident in acute cases of dialysis imbalance syndrome. Another key factor may be residual renal function, which many PD patients typically retain compared to HD patients, resulting in better hemodynamic stability and better clinical outcomes [42,43]. PD patients in our study also have better residual renal function than HD patients, which could partly justify better CRIq results.
Another factor can be better removal of intermediate molecules with PD compared to HD [44,45].
Furthermore, having free mornings for work and exercise in individuals with PD improves overall mental status, including cognition and prevention of depression [46].
Limitations of this study
A possible selection bias of the population analyzed and its retrospective nature. Indeed, the different CRIq scores between PD patients, HD patients, and controls may be due to different cognitive scores at baseline. A possible future study could be set up prospectively, enrolling patients in PD and HD and controls with an equivalent cognitive score at baseline and repeating the questionnaire after at least one year of dialysis treatment.
CI represents an important problem during chronic kidney disease and dialysis therapy, whatever the method used, also involving non-elderly patients. It seems, in fact, that cognitive problems may occur in patients undergoing PD earlier and with a greater frequency than in the general population, but with a lower incidence than in patients on HD.
These considerations should be communicated to patients when they are educated about different replacement methods: many patients choose, in fact, to undergo HD which can be harmful to long-term cognition without a thorough discussion with healthcare professionals regarding the decline in function cognitive.
In summary, while further randomized controlled trials are needed to confirm these findings, our findings have the potential to impact the counseling of future renal patients and their families when considering the choice of dialysis method.
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015 Jun;11(6):718-26. doi: 10.1016/j.jalz.2015.05.016. Epub 2015 Jun 1. PMID: 26045020.
- Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, Buot V, Murray AM. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis. 2011 Apr;57(4):612-20. doi: 10.1053/j.ajkd.2010.11.026. Epub 2011 Feb 4. Erratum in: Am J Kidney Dis. 2011 Jun;57(6):966. PMID: 21295896; PMCID: PMC3121243.
- Collins AJ, Li S, Ma JZ, Herzog C. Cardiovascular disease in end-stage renal disease patients. Am J Kidney Dis. 2001 Oct;38(4 Suppl 1):S26-9. doi: 10.1053/ajkd.2001.27392. PMID: 11576917.
- Levin A. Clinical epidemiology of cardiovascular disease in chronic kidney disease prior to dialysis. Semin Dial. 2003 Mar-Apr;16(2):101-5. doi: 10.1046/j.1525-139x.2003.16025.x. PMID: 12641872.
- Olczyk P, Kusztal M, Gołębiowski T, Letachowicz K, Krajewska M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. Int J Environ Res Public Health. 2022 Feb 18;19(4):2389. doi: 10.3390/ijerph19042389. PMID: 35206577; PMCID: PMC8877881.
- USRDS. Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. U.S. Renal Data System. Bethesda MD. 2005.
- Kim CD, Lee HJ, Kim DJ, Kim BS, Shin SK, Do JY, Jang MH, Park SH, Kim YS, Kim YL. High prevalence of leukoaraiosis in cerebral magnetic resonance images of patients on peritoneal dialysis. Am J Kidney Dis. 2007 Jul;50(1):98-107. doi: 10.1053/j.ajkd.2007.03.019. PMID: 17591529.
- Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG; MRC Cognitive Function and Ageing Neuropathology Study Group. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006 Jun;37(6):1391-8. doi: 10.1161/01.STR.0000221308.94473.14. Epub 2006 Apr 20. PMID: 16627790.
- Schmidt R, Petrovic K, Ropele S, Enzinger C, Fazekas F. Progression of leukoaraiosis and cognition. Stroke. 2007 Sep;38(9):2619-25. doi: 10.1161/STROKEAHA.107.489112. Epub 2007 Aug 2. PMID: 17673724.
- Inzitari D, Pracucci G, Poggesi A, Carlucci G, Barkhof F, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Pantoni L; LADIS Study Group. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009 Jul 6;339:b2477. doi: 10.1136/bmj.b2477. PMID: 19581317; PMCID: PMC2714680.
- Khawar O, Kalantar-Zadeh K, Lo WK, Johnson D, Mehrotra R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol. 2007 Nov;2(6):1317-28. doi: 10.2215/CJN.02550607. Epub 2007 Oct 17. PMID: 17942769.
- Cereda E, Sacchi MC, Malavazos AE. Central obesity and increased risk of dementia more than three decades later. Neurology. 2009 Mar 17;72(11):1030-1; author reply 1031. doi: 10.1212/01.wnl.0000343499.72241.ea. PMID: 19289749.
- Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009 Mar;66(3):324-8. doi: 10.1001/archneurol.2008.566. PMID: 19273750; PMCID: PMC2685462.
- Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, Yaffe K; Health ABC Study. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol. 2009 Mar;66(3):329-35. doi: 10.1001/archneurol.2008.570. PMID: 19273751; PMCID: PMC2739693.
- Wu PH, Lin YT, Wu PY, Huang JC, Chen SC, Chang JM, Chen HC. A Low Ankle-Brachial Index and High Brachial-Ankle Pulse Wave Velocity Are Associated with Poor Cognitive Function in Patients Undergoing Hemodialysis. Dis Markers. 2019 Aug 19;2019:9421352. doi: 10.1155/2019/9421352. PMID: 31531128; PMCID: PMC6721107.
- Tasmoc A, Donciu MD, Veisa G, Nistor I, Covic A. Increased arterial stiffness predicts cognitive impairment in hemodialysis patients. Hemodial Int. 2016 Jul;20(3):463-72. doi: 10.1111/hdi.12406. Epub 2016 Feb 9. PMID: 26861856.
- Liu W, Wang L, Huang X, Yuan C, Li H, Yang J. Orthostatic blood pressure reduction as a possible explanation for memory deficits in dialysis patients. Hypertens Res. 2019 Jul;42(7):1049-1056. doi: 10.1038/s41440-019-0236-4. Epub 2019 Mar 1. PMID: 30824825.
- Bossola M, Tazza L, Vulpio C, Luciani G. Is regression of left ventricular hypertrophy in maintenance hemodialysis patients possible? Semin Dial. 2008 Sep-Oct;21(5):422-30. doi: 10.1111/j.1525-139X.2008.00471.x. Epub 2008 Aug 28. PMID: 18764802.
- Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001 May;12(5):1079-1084. doi: 10.1681/ASN.V1251079. PMID: 11316868.
- Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab. 2007 Nov;27(11):1861-9. doi: 10.1038/sj.jcbfm.9600478. Epub 2007 Apr 4. PMID: 17406658.
- Bossola M, Laudisio A, Antocicco M, Tazza L, Colloca G, Tosato M, Zuccalà G. Cognitive performance is associated with left ventricular function in older chronic hemodialysis patients: result of a pilot study. Aging Clin Exp Res. 2014 Aug;26(4):445-51. doi: 10.1007/s40520-013-0191-x. Epub 2013 Dec 19. PMID: 24353108.
- MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J Am Soc Nephrol. 2017 Aug;28(8):2511-2520. doi: 10.1681/ASN.2016060704. Epub 2017 Mar 7. PMID: 28270412; PMCID: PMC5533227.
- Polinder-Bos HA, García DV, Kuipers J, Elting JWJ, Aries MJH, Krijnen WP, Groen H, Willemsen ATM, van Laar PJ, Strijkert F, Luurtsema G, Slart RHJA, Westerhuis R, Gansevoort RT, Gaillard CAJM, Franssen CFM. Hemodialysis Induces an Acute Decline in Cerebral Blood Flow in Elderly Patients. J Am Soc Nephrol. 2018 Apr;29(4):1317-1325. doi: 10.1681/ASN.2017101088. Epub 2018 Mar 1. Erratum in: J Am Soc Nephrol. 2018 Aug;29(8):2256. PMID: 29496888; PMCID: PMC5875962.
- Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, Smith AC. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol. 2014 Sep;25(9):2121-30. doi: 10.1681/ASN.2013070702. Epub 2014 Apr 3. PMID: 24700875; PMCID: PMC4147973.
- Carlsson AC, Carrero JJ, Stenvinkel P, Bottai M, Barany P, Larsson A, Ärnlöv J. Endostatin, Cathepsin S, and Cathepsin L, and Their Association with Inflammatory Markers and Mortality in Patients Undergoing Hemodialysis. Blood Purif. 2015;39(4):259-65. doi: 10.1159/000381664. Epub 2015 Apr 29. PMID: 25924922.
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013 Feb;39(1):19-34. doi: 10.1111/j.1365-2990.2012.01306.x. PMID: 23039106; PMCID: PMC3553257.
- Prelevic V, Radunovic D, Antunovic T, Ratkovic M, Gligorovic-Bahranovic N, Gledovic B, Vujosevic S, Nedovic-Vukovic M, Basic-Jukic N. Increased Serum Level of IGF-1 Correlates With Better Cognitive Status in End-Stage Renal Disease Patients Undergoing Hemodialysis. Ther Apher Dial. 2018 Apr;22(2):118-123. doi: 10.1111/1744-9987.12610. Epub 2017 Dec 7. PMID: 29214734.
- Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009 May 19;119(19):2545-52. doi: 10.1161/CIRCULATIONAHA.108.844506. Epub 2009 May 4. PMID: 19414634; PMCID: PMC2740903.
- Kirkpantur A. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2010.
- Josipa R. Is There Differences in Cognitive and Motor Functioning between Hemodialysis and Peritoneal Dialysis Patients? Renal Failure.
- Wolcott DL, Wellisch DK, Marsh JT, Schaeffer J, Landsverk J, Nissenson AR. Relationship of dialysis modality and other factors to cognitive function in chronic dialysis patients. Am J Kidney Dis. 1988 Oct;12(4):275-84. doi: 10.1016/s0272-6386(88)80220-8. PMID: 3177371.
- Buoncristiani U, Alberti A, Gubbiotti G, Mazzotta G, Gallai V, Quintaliani G, Gaburri M. Better preservation of cognitive faculty in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1993;13 Suppl 2:S202-5. PMID: 8399566.
- Tilki HE, Akpolat T, Tunali G, Kara A, Onar MK. Effects of haemodialysis and continuous ambulatory peritoneal dialysis on P300 cognitive potentials in uraemic patients. Ups J Med Sci. 2004;109(1):43-8. doi: 10.3109/2000-1967-109. PMID: 15124952.
- Kutlay S, Nergizoglu G, Duman N, Atli T, Keven K, Ertürk S, Ates K, Karatan O. Recognition of neurocognitive dysfunction in chronic hemodialysis patients. Ren Fail. 2001 Nov;23(6):781-7. doi: 10.1081/jdi-100108189. PMID: 11777317.
- Sithinamsuwan P, Niyasom S, Nidhinandana S, Supasyndh O. Dementia and depression in end stage renal disease: comparison between hemodialysis and continuous ambulatory peritoneal dialysis. J Med Assoc Thai. 2005 Nov;88 Suppl 3:S141-7. PMID: 16858952.
- Lai S, Mecarelli O, Pulitano P, Romanello R, Davi L, Zarabla A, Mariotti A, Carta M, Tasso G, Poli L, Mitterhofer AP, Testorio M, Frassetti N, Aceto P, Galani A, Lai C. Neurological, psychological, and cognitive disorders in patients with chronic kidney disease on conservative and replacement therapy. Medicine (Baltimore). 2016 Nov;95(48):e5191. doi: 10.1097/MD.0000000000005191. PMID: 27902586; PMCID: PMC5134816.
- Lambert K, Mullan J, Mansfield K, Lonergan M. Comparison of the extent and pattern of cognitive impairment among predialysis, dialysis and transplant patients: A cross-sectional study from Australia. Nephrology (Carlton). 2017 Nov;22(11):899-906. doi: 10.1111/nep.12892. PMID: 27505310.
- Neumann D, Robinski M, Mau W, Girndt M. Cognitive Testing in Patients with CKD: The Problem of Missing Cases. Clin J Am Soc Nephrol. 2017 Mar 7;12(3):391-398. doi: 10.2215/CJN.03670316. Epub 2017 Feb 1. PMID: 28148556; PMCID: PMC5338701.
- Iyasere O, Okai D, Brown E. Cognitive function and advanced kidney disease: longitudinal trends and impact on decision-making. Clin Kidney J. 2017 Feb;10(1):89-94. doi: 10.1093/ckj/sfw128. Epub 2017 Jan 7. PMID: 28638609; PMCID: PMC5469575.
- Neumann D, Mau W, Wienke A, Girndt M. Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int. 2018 Feb;93(2):430-438. doi: 10.1016/j.kint.2017.07.022. Epub 2017 Oct 14. PMID: 29042081.
- Tian X, Guo X, Xia X, Yu H, Li X, Jiang A. The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019 Feb;98(6):e14390. doi: 10.1097/MD.0000000000014390. PMID: 30732180; PMCID: PMC6380759.
- Zsom L, Zsom M, Fulop T, Flessner MF. Treatment time, chronic inflammation, and hemodynamic stability: the overlooked parameters in hemodialysis quantification. Semin Dial. 2008 Sep-Oct;21(5):395-400. doi: 10.1111/j.1525-139X.2008.00488.x. PMID: 18945325.
- Zsom L, Zsom M, Abdul Salim S, Fülöp T. Subjective global assessment of nutrition, dialysis quality, and the theory of the scientific method in Nephrology practice. Artif Organs. 2020 Oct;44(10):1021-1030. doi: 10.1111/aor.13762. Epub 2020 Jul 27. PMID: 33617092.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283(15):2008-12. doi: 10.1001/jama.283.15.2008. PMID: 10789670.
- Li Y, Pi HC, Yang ZK, Dong J. Associations between small and middle molecules clearance and the change of cognitive function in peritoneal dialysis. J Nephrol. 2019.
- Levendoğlu F, Altintepe L, Okudan N, Uğurlu H, Gökbel H, Tonbul Z, Güney I, Türk S. A twelve-week exercise program improves the psychological status, quality of life and work capacity in hemodialysis patients. J Nephrol. 2004 Nov-Dec;17(6):826-32. PMID: 15593058.