More Information
Submitted: July 06, 2023 | Approved: July 27, 2023 | Published: July 28, 2023
How to cite this article: Mahoney MS, Kincaide EL, Nelson J, Klein KA, Hall RC, et al. Evaluation of a Weight-Based Mycophenolate Mofetil Dosing Protocol for Kidney Transplant Maintenance Immunosuppression. J Clini Nephrol. 2023; 7: 047-052.
DOI: 10.29328/journal.jcn.1001108
Copyright License: © 2023 Mahoney MS, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: MMF: Mycophenolate Mofetil; KTR: Kidney Transplant Recipients; BPAR: Biopsy-Proven Acute Rejection; G-CSF: Granulocyte Colony-Stimulating Factor; MPA: Mycophenolic Acid; AUC: Area Under The Curve; DGF: Delayed Graft Function; CMV: Cytomegalovirus; EBV: Epstein Barr Virus; ACR: Acute Cellular Rejection; AMR: Antibody-Mediated Rejection
Evaluation of a Weight-Based Mycophenolate Mofetil Dosing Protocol for Kidney Transplant Maintenance Immunosuppression
Melanie Tess Mahoney1,2* , Elisabeth Lapp Kincaide1,2
, Elisabeth Lapp Kincaide1,2 , Joelle Nelson1,2
, Joelle Nelson1,2 , Kelsey Anne Klein1,2
, Kelsey Anne Klein1,2 , Reed Charles Hall1,2
, Reed Charles Hall1,2 and Suverta Bhayana2
and Suverta Bhayana2
1University Health, The University of Texas at Austin, College of Pharmacy, Pharmacotherapy Division, Austin, TX, USA
2The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
*Address for Correspondence: Melanie Tess Mahoney, University Health, The University of Texas at Austin, College of Pharmacy, Pharmacotherapy Division, Austin, TX; The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA, Email: [email protected]
To evaluate the safety and efficacy of weight-based mycophenolate mofetil (MMF) dosing in adult kidney transplant recipients (KTR), this single-center retrospective study of adult KTR compared biopsy-proven acute rejection (BPAR), infections, hospitalizations, granulocyte colony-stimulating factor (G-CSF) use, and MMF dose changes within one year of transplant pre-and post-implementation of a weight-based MMF dosing protocol. Adult patients who received a kidney transplant at University Health Transplant Institute were reviewed for inclusion. Patients in the weight-based MMF group received 1000 mg twice daily by the first clinic visit if ≥ 80 kg, 750 mg twice daily if 50-79 kg, and 500 mg twice daily if < 50 kg. Patients in the fixed-dose MMF group received MMF 1000 mg twice daily. A total of 170 KTR (50.0% ≥ 80 kg, 44.1% 50-79 kg, 5.9% < 50 kg) were included. Baseline characteristics were similar between groups. The majority of patients were middle-aged Hispanic males and received lymphocyte-depleting induction therapy. Incidences of BPAR, infection, and hospitalization were similar between both groups at one-year post-transplant. Weight-based MMF dosing is safe and effective in adult KTR.
Mycophenolate Mofetil (MMF) is an anti-proliferative agent commonly used for maintenance immunosuppression post-transplant. In 2019, 93% of adult KTR in the United States received MMF as part of their initial immunosuppression regimen. The most common regimens currently are MMF, tacrolimus, and a corticosteroid (65% of patients) followed by MMF and tacrolimus (28% of patients) [1]. MMF is recommended to be administered at a dose of 1,000 mg by mouth twice daily for adult KTR [2]. In clinical practice, this dose often needs to be decreased due to intolerance such as gastrointestinal adverse effects and leukopenia [3-5].
MMF is a prodrug that is hydrolyzed to its active metabolite Mycophenolic Acid (MPA) upon absorption. MPA exposure has been demonstrated to correlate with MMF dose per kilogram of total body weight [6]. This suggests that a fixed dose of MMF may not be appropriate for all patients, notably those who are under or over-weight. Therapeutic drug monitoring of MMF, primarily in combination with cyclosporine, has demonstrated a target MPA area under the curve (AUC) of > 30 mg·h/L is associated with decreased incidence of biopsy-proven acute rejection (BPAR) and an AUC of < 60 mg·h/L is associated with decreased leukopenia [7,8]. It is important to note, however, that concomitant cyclosporine use is associated with decreased MPA exposure due to cyclosporine inhibiting the transport of MPA’s primary metabolite, a pharmacologically inactive glucuronide (MPAG) [8]. In clinical practice, therapeutic drug monitoring of MMF is not routinely recommended due to the cost and time required in addition to minimal evidence showing clinical benefit [9].
Pharmacokinetic studies have investigated the dose of MMF (based on body weight) needed to achieve therapeutic concentrations. A 2007 study measured multiple MPA serum concentrations in 53 Asian KTR receiving cyclosporine and corticosteroid in addition to MMF immunosuppression. The study found that an MMF dose of 12 mg/kg twice daily using total body weight could be used to achieve an MPA AUC of 45 mg·h/L [6]. Another study measured MPA serum concentrations in 43 Japanese KTR that a dose of 10-16 mg/kg twice daily could predict a mycophenolate AUC of 30 - 60 mg·h/l with a probability of 75% [10]. Neither pharmacokinetic study analyzed clinical outcomes such as cytopenia and gastrointestinal adverse effects. Further reports suggest that doses less than 2000 mg/day may be better tolerated with no change in efficacy. A prospective Iranian study compared 22 KTR receiving reduced-dose MMF to 33 patients receiving fixed-dose MMF, evaluating allograft rejection rates and impaired allograft function rates. There were no significant differences in these outcomes between the two groups [11]. The Opticept trial post hoc weight analysis found an inverse relationship between MPA AUC and weight. Notably, the patients included in this study received tacrolimus with MMF. These authors concluded that patients at weight extremes may be at risk of under or overimmunosuppression with fixed-dose MMF [12]. Pharmacokinetic data have demonstrated what weight-based MMF doses can achieve goal AUC and that fixed-dose MMF may not be appropriate for all patients. Additionally, clinical data have produced mixed results about the safety of reducing MMF doses due to adverse effects. There is a lack of data reporting clinical outcomes associated with weight-based MMF dosing initially post-transplant.
This study sought to evaluate outcomes associated with the transition from an MMF fixed-dose protocol to a weight-based dosing protocol that has been used by University Health Transplant Institute since August 30, 2018. Outcomes from KTR who received weight-based MMF were compared to a historical cohort of KTR immediately prior to protocol implementation who received fixed-dose MMF.
Patient sample
All patients > 18 years of age who received a kidney transplant at UTC between June 28, 2016, and May 1, 2020, were evaluated for inclusion in the study. Exclusion criteria included delayed graft function (DGF; dialysis required within seven days of transplant [13]), slow graft function (SGF; 24-hour urine output ≤ 500 mL by postoperative day 2), non-lymphocyte induction therapy, Black race, previous transplant, and multi-organ transplant. All exclusion criteria match exclusion criteria for the center’s current weight-based MMF protocol to ensure similar baseline characteristics between the weight-based and fixed-dose MMF cohorts. Additionally, patients who received MMF doses that did not match the institutional protocol were excluded from the study. Patients were included in either a weight-based or fixed-dose MMF cohort based on their dose at discharge from index admission.
Immunosuppression
The patient received induction immunosuppression with either alemtuzumab 30 mg intraoperatively or rabbit anti-thymocyte globulin (rATG) cumulative weight-adjusted dose of 4.5 mg/kg per institutional protocol. Patients considered to have high immunologic risk (pre-formed donor-specific antibody or DSA, repeat kidney transplant, Black race, positive cross-match, or inflammatory glomerulonephritis) received rATG. Patients considered to have standard immunologic risk (non-HLA identical, highly sensitized with no DSA, repeat kidney transplant with no DSA, or noninflammatory glomerulonephritis) received alemtuzumab. Those at low immunologic risk (not sensitized, no pre-formed DSA, ≥70 years of age) received basiliximab induction and were not included in this study. Percenter protocol, patients receive maintenance immunosuppression indefinitely with tacrolimus (goal trough 8-12 ng/mL for three months after transplant, 6 - 8 ng/mL for months 4 - 12 post-transplant, and 4 - 6 ng/mL for the time greater than one-year post-transplant), prednisone tapered to 5 mg by Postoperative Day (POD) 5 and MMF post kidney transplant. Immediately post-transplant, all patients in the study received MMF 1000 mg PO BID until the day of discharge. On the day of discharge, patients in the weight-based cohort had MMF doses adjusted as follows: those weighing greater than or equal to 80 kg received 1000 mg by mouth twice daily (BID), patients 50 to 79 kg received 750 mg by mouth BID, and patients weighing less than 50 kg received 500 mg by mouth BID. This dosing scheme is equivalent to patients receiving approximately 10 - 15 mg/kg/dose MMF. Patients in the fixed-dose cohort all continued to receive MMF 1000 mg by mouth BID upon discharge. All patients received surgical infection prophylaxis, Pneumocystis jirovecii pneumonia prophylaxis for 6 months, and serology-stratified cytomegalovirus prophylaxis per institutional protocol (CMV high-risk patients received valganciclovir 450 mg twice daily for 6 months, CMV moderate risk patients received valganciclovir 450 mg once daily for 3 months, and CMV low-risk patients received acyclovir 400 mg twice daily for 3 months).
Outcomes
Outcomes evaluated include BPAR incidence, infection, all-cause hospitalization, hospitalization reason, G-CSF use, leukopenia, neutropenia, and MMF dose changes. BPAR included Antibody-Mediated Rejection (AMR) or Acute Cellular Rejection (ACR) with a Banff grade of 1A or greater. Infection included any positive Cytomegalovirus (CMV) PCR, Epstein Barr virus (EBV) PCR, BK virus plasma quantitative test, stool PCR panel, viral panel, fungal serology, or bacterial or fungal culture. Leukopenia was defined as a white blood cell count < 4000 cells/µL. Neutropenia was defined as an Absolute Neutrophil Count (ANC) < 1500 cells/µL and severe neutropenia was defined as an ANC < 500 cells/µL. All outcomes were evaluated within one-year post-transplant.
Statistical analysis
Outcomes were analyzed via chi-square testing and two-sided Fischer’s exact testing as appropriate for categorical variables and Mann-Whitney U testing was used for continuous variables. JMP 14 was used for statistical analysis.
This study was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board.
Baseline characteristics
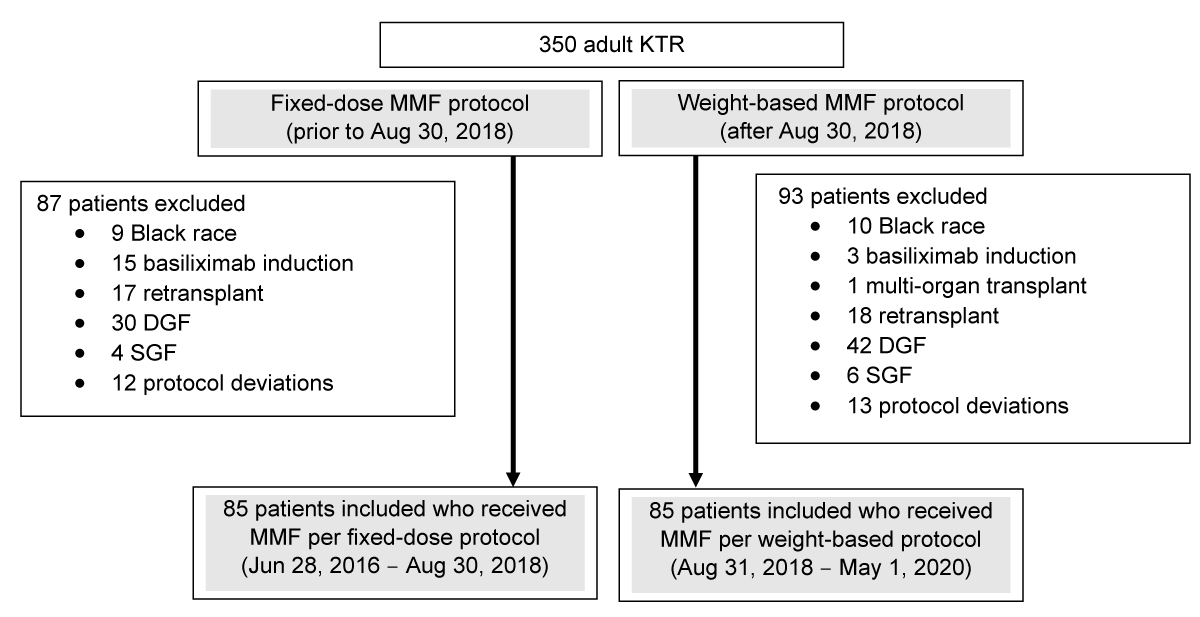
A total of 350 KTRs were reviewed for inclusion in the study. After the exclusion of 180 KTR, a total of 170 were included in the study. Exclusion reasons are shown in Figure 1, with the most common being DGF status. Eighty-five patients transplanted between August 31, 2018, and May 1, 2020, who received weight-based MMF were compared to a retrospective cohort of 85 patients transplanted between June 28, 2016, and August 30, 2018, who received MMF 1000 mg BID (fixed-dose). Patients in the fixed-dose group tended to be slightly older (median age 51 years vs. 48 years; p = 0.04) and there were fewer patients considered to be Public Health Service (PHS) high-risk among the fixed-dose cohort than the weight-based MMF cohort (15.3% vs. 28.2%; p = 0.04). Otherwise, baseline characteristics were similar between both cohorts and are described in Table 1. Most patients were middle-aged (median 50 years) Hispanic (63.5%) males (60.6%) and received rabbit anti-thymocyte globulin (58.8%) for induction therapy.
Figure 1: Flow diagram. This diagram describes the study population. A total of 350 adult KTRs were reviewed for inclusion. Among patients transplanted prior to August 30, 2018 (fixed-dose MMF protocol), a total of 87 patients were excluded. Among patients transplanted after August 30, 2018 (weight-based MMF protocol), a total of 93 patients were excluded.
| Table 1: Baseline characteristics. | |||
| Fixed-dose MMF N = 85 |
Weight-based MMF N = 85 |
p - value | |
| Male, n (%) | 49 (57.7) | 54 (63.5) | 0.43 |
| Age, median in years (IQR) | 51 (45-59) | 48 (38-56) | 0.04 |
| Ethnicity, n (%) | 0.18 | ||
| Hispanic white | 53 (62.4) | 55 (64.7) | |
| Non-Hispanic white | 32 (37.6) | 27 (31.8) | |
| Asian | 0 (0) | 3 (3.5) | |
| Donor type, n (%) | 0.28 | ||
| Living donor | 34 (40.0) | 41 (48.2) | |
| Deceased donor | 51 (60.0) | 44 (57.6) | |
| Etiology of kidney disease, n (%)† |
0.54 1.00 0.72 0.35 |
||
| Diabetes mellitus | 37 (43.5) | 41 (48.2) | |
| Glomerular nephritis | 24 (28.2) | 24 (28.2) | |
| Hypertension | 21 (24.7) | 19 (22.4) | |
| Other | 4 (4.7) | 7 (8.2) | |
| Weight category, n (%) | 0.54 | ||
| <50 kg | 3 (3.5) | 2 (2.4) | |
| 50-79 kg | 43 (50.6) | 37 (43.5) | |
| ≥80 kg | 39 (45.9) | 46 (54.1) | |
| Induction immunosuppression, n (%) | 0.53 | ||
| Alemtuzumab | 33 (38.8) | 37 (43.5) | |
| Rabbit anti-thymocyte globulin | 52 (61.2) | 48 (56.5) | |
| PHS, n (%) | 0.04 | ||
| High risk | 13 (15.3) | 24 (28.2) | |
| Not high risk | 72 (84.7) | 61 (71.8) | |
| CMV risk, n (%) | 0.24 | ||
| Low (D-/R-) | 9 (10.6) | 4 (4.7) | |
| Moderate (R+) | 60 (70.6) | 59 (69.4) | |
| High (D+/R-) | 16 (18.8) | 22 (25.9) | |
Outcomes
There were no significant differences in outcomes between cohorts. Rates of BPAR within one year of transplant were similar between groups with 12.9% in the fixed-dose group and 15.3% in the weight-based group (p = 0.66). Two patients who received fixed-dose MMF and three who received weight-based MMF experienced ACR with a Banff grade of 2. Similarly, there was no difference in the incidence of any infection within one year (76.5% vs. 83.5%; p = 0.25). There were also no significant differences in incidences of different types of infections (CMV, EBV, BK viremia, Hepatitis C, bacteriuria, bacterial pneumonia, bacteremia, fungal pneumonia, fungemia, or Clostridioides difficile). Myelotoxicity was similar between groups. Of note, there were six incidences of Hepatitis C in the weight-based group, likely due to a change in center practice at that time to accept HCV-positive donors. While there were numerically more incidences of severe neutropenia in the fixed-dose MMF group, this difference was not statistically significant (14.1% vs. 8.2%; p = 0.22). G-CSF use was similar between groups (23.5% vs. 17.6%, p = 0.34). Rates of all-cause hospitalization within one year were also similar (55.3% vs. 48.2%;
p = 0.36). When reviewing hospitalization reasons, there were numerically more incidences of hospitalization associated with GI ADEs in the fixed-dose MMF group (10.6% vs. 3.5%; p = 0.08). Rates of MMF dose decreases were numerically greater among those patients in the fixed-dose cohort, although this difference was not statistically significant (83.5% vs. 71.7%; p = 0.10). Complete outcomes are shown in Table 2.
| Table 2: Outcomes at 1-year post-transplant. | |||
| Fixed-dose MMF, n (%) N = 85 |
Weight-based MMF, n (%) N = 85 |
p - value | |
| BPAR | 11 (12.9) | 13 (15.3) | 0.66 |
| AMR | 1 (1.2) | 4 (4.7) | 0.37 |
| ACR | 11 (12.9) | 11 (12.9) | 1.00 |
| 1A | 4 (4.7) | 5 (5.9) | 1.00 |
| 1B | 5 (5.9) | 3 (3.5) | 0.72 |
| 2A | 1 (1.2) | 3 (3.5) | 1.00 |
| 2B | 1 (1.2) | 0 | 1.00 |
| Any infection | 65 (76.5) | 71 (83.5) | 0.25 |
| CMV | 20 (23.5) | 23 (27.1) | 0.72 |
| EBV | 0 (0) | 1 (1.2) | 1.00 |
| BK | 29 (34.1) | 32 (37.6) | 0.75 |
| HepC | 0 (0) | 6 (7.1) | 0.03 |
| Bacteriuria | 45 (52.9) | 37 (43.5) | 0.28 |
| Bacterial pneumonia | 4 (4.7) | 4 (4.7) | 1.00 |
| Bacteremia | 7 (8.2) | 10 (11.8) | 0.61 |
| Fungal pneumonia | 2 (2.4) | 1 (1.2) | 1.00 |
| Fungemia | 0 (0) | 2 (2.4) | 0.50 |
| Clostridioides difficile | 6 (7.1) | 1 (1.2) | 0.12 |
| Leukopenia (WBC < 4) | 60 (70.6) | 59 (69.4) | 0.87 |
| Neutropenia (ANC < 1500) | 28 (32.9) | 33 (38.8) | 0.42 |
| Moderate-severe neutropenia (ANC < 1000) | 24 (28.2) | 20 (23.5) | 0.48 |
| Severe neutropenia (ANC < 500) | 12 (14.1) | 7 (8.2) | 0.22 |
| G-CSF use | 20 (23.5) | 15 (17.6) | 0.34 |
| MMF dose decrease | 71 (83.5) | 61 (71.7) | 0.10 |
| Due to GI ADEs | 10 (11.8) | 14 (16.5) | 0.51 |
| Due to leukopenia | 22 (25.9) | 21 (24.7) | 1.00 |
| Due to infection | 29 (34.1) | 24 (28.2) | 0.51 |
| Due to other/unknown | 9 (10.6) | 2 (2.4) | 0.06 |
| MMF dose increase | 0 | 3 (3.5) | 0.25 |
| Hospitalization | 47 (55.3) | 41 (48.2) | 0.36 |
| Associated with neutropenia | 3 (3.5) | 3 (3.5) | 1.00 |
| Associated with any infection | 31 (36.5) | 31 (36.5) | 1.00 |
| Associated with GI ADEs | 9 (10.6) | 3 (3.5) | 0.08 |
A subgroup analysis was conducted to assess outcomes among patients weighing < 80 kg as these patients received decreased doses of MMF per the weight-based protocol. Among this subgroup analysis, there were no significant differences in outcomes between patients who received reduced-dose MMF per the weight-based protocol and those who received fixed-dose MMF. Rates of BPAR remained similar between cohorts (13.0% vs. 15.4%; p = 0.77), as did the incidence of infection (73.9% vs. 82.1%; p = 0.37) and hospitalization (52.2% vs. 43.6%; p = 0.43). In this subgroup analysis, there were significantly fewer MMF dose decreases required in the weight-based MMF cohort (59.0% vs. 84.8%; p = 0.01). Three patients in the weight-based cohort had their MMF dose increased due to rejection. All patients survived one-year post-transplant. Only one patient experienced graft loss in this study. This individual was in the weight-based cohort but was >80 kg so received MMF 1000 mg BID. All patients reviewed in this study were alive at 1 year post-transplant. Additional outcomes are shown in Table 3. Among patients >65 years of age, 11 out of 13 required a dose decrease of MMF, most frequently (5 patients) due to infection.
| Table 3: Outcomes at 1-year post-transplant among patients weighing < 80 kg. | |||
| Fixed-dose MMF, n (%) N = 46 |
Weight-based MMF, n (%) N = 39 |
p - value | |
| BPAR | 6 (13.0) | 6 (15.4) | 0.77 |
| AMR | 1 (2.2) | 2 (5.1) | 0.59 |
| ACR | 6 (13.0) | 6 (15.4) | 0.77 |
| 1A | 2 (4.3) | 3 (7.7) | 0.66 |
| 1B | 3 (6.5) | 2 (5.1) | 1 |
| 2A | 0 | 1 (2.6) | 0.46 |
| 2B | 1 (2.2) | 0 | 1 |
| Any infection | 34 (73.9) | 32 (82.1) | 0.37 |
| CMV | 10 (21.7) | 9 (23.1) | 1.00 |
| EBV | 0 (0) | 0 (0) | 1.00 |
| BK | 13 (28.3) | 15 (38.5) | 0.36 |
| HepC | 0 (0) | 0 (0) | 1.00 |
| Bacteriuria | 25 (54.3) | 19 (48.7) | 0.67 |
| Bacterial pneumonia | 1 (2.2) | 0 (0) | 1.00 |
| Bacteremia | 6 (13.0) | 5 (12.8) | 1.00 |
| Fungal pneumonia | 0 (0) | 0 (0) | 1.00 |
| Fungemia | 0 (0) | 1 (2.6) | 0.46 |
| Clostridioides difficile | 2 (4.3) | 0 (0) | 0.50 |
| Leukopenia (WBC <4) | 34 (73.9) | 27 (69.2) | 0.63 |
| Neutropenia (ANC <1500) | 16 (34.8) | 14 (35.9) | 0.91 |
| Moderate-severe neutropenia (ANC <1000) | 13 (28.3) | 8 (20.5) | 0.41 |
| Severe neutropenia (ANC <500) | 5 (10.9) | 4 (10.3) | 1.00 |
| G-CSF use | 10 (21.7) | 6 (15.4) | 0.46 |
| MMF dose decrease | 39 (84.8) | 23 (59.0) | 0.01 |
| Due to GI ADEs | 7 (15.2) | 5 (12.8) | 1.00 |
| Due to leukopenia | 8 (17.4) | 8 (20.5) | 0.78 |
| Due to infection | 15 (32.6) | 10 (25.6) | 0.63 |
| Due to other/unknown | 8 (17.4) | 0 | 0.01 |
| MMF dose increase | 0 | 3 (7.7) | 0.09 |
| Hospitalization | 24 (52.2) | 17 (43.6) | 0.43 |
| Associated with neutropenia | 1 (2.2) | 2 (5.1) | 0.59 |
| Associated with any infection | 16 (34.8) | 14 (35.9) | 0.91 |
| Associated with GI ADEs | 4 (8.7) | 2 (5.1) | 0.68 |
There is currently a paucity of data on weight-based approaches to dosing of MMF in KTR. A previously-published abstract reported an increased incidence of BPAR in high immunologic risk patients receiving low-dose of mycophenolic acid relative to body weight (defined as < 20 mg/kg/day). These findings suggest that fixed doses without regard to patient weight may be inappropriate and weight-based approaches should be pursued [14]. Previous studies have reported an increased incidence of acute rejection when MMF doses were decreased due to protocol or adverse effects [15]. Results from the present study support that weight-based MMF dosing is safe and effective in a select subgroup of KTR who are at moderate to high immunologic risk and received lymphocyte-depleting induction therapy. Patients who experience DGF or SGF, receive non-lymphocyte induction therapy, are of black race, or have received a previous transplant have been associated with a greater risk of rejection [16-22]. These patients, as well as multi-organ transplant recipients, are presently excluded from the institution’s weight-based MMF protocol and thus these results cannot be extrapolated to these populations.
To our knowledge, this is the first study to report clinical outcomes associated with a weight-based MMF protocol for KTR. Another strength of this study includes the large Hispanic patient population, which has historically been underrepresented in clinical trials. Previous reports of safety with weight-based MMF post-transplant have come from primarily Asian populations [5,6].
This study is limited by its retrospective nature. The use of a retrospective cohort introduces the potential for confounders. Despite this limitation, groups were well-matched in baseline characteristics. The small sample size when performing the subgroup analysis for those patients weighing <80 kg, also limits the analysis and increases the potential for type II error in the patient populations that a decreased dose of MMF is most likely to benefit. Additionally, the study was limited to one year of follow-up. As all patients received lymphocyte-depleting induction therapy, an extended follow-up may allow for a better analysis of long-term outcomes impacted by MMF dosing such as rejection. This study also did not assess for patient-reported adverse effects, such as gastrointestinal adverse drug effects (GI ADEs), that are frequently reported with MMF [23-26]. In lieu of patient-reported adverse effects, MMF dose changes and hospitalizations associated with GI ADEs were reported, and no difference was found.
The results from this study demonstrate that a weight-based approach to MMF dosing is likely safe for moderate to high immunologic risk KTR without additional risk factors. There may be certain populations that could benefit most from a decreased dose of MMF such as those with a lower immunologic risk or older age, which need to be further elucidated. Additional data are needed, ideally in a prospective study with therapeutic drug monitoring while assessing MMF-associated ADEs and BPAR, to determine the best approach to MMF dosing post-kidney transplant.
- Hart A, Lentine K, Smith J. OPTN/SRTR 2019 Annual Data Report: Kidney. 2019: 105.
- Genentech USA. Cellcept (mycophenolate mofetil): Highlights of Prescribing Information. San Francisco, CA. 1995.
- Kahu J, Kyllönen L, Salmela K. Impact of mycophenolate mofetil intolerance on early results of kidney transplantation. Transplant Proc. 2005 Oct;37(8):3276-9. doi: 10.1016/j.transproceed.2005.09.014. PMID: 16298571.
- Squifflet JP, Bäckman L, Claesson K, Dietl KH, Ekberg H, Forsythe JL, Kunzendorf U, Heemann U, Land W, Morales JM, Mühlbacher F, Talbot D, Taube D, Tyden G, van Hooff J, Schleibner S, Vanrenterghem Y; European Tacrolimus-MMF Renal Study Group. Dose optimization of mycophenolate mofetil when administered with a low dose of tacrolimus in cadaveric renal transplant recipients. Transplantation. 2001 Jul 15;72(1):63-9. doi: 10.1097/00007890-200107150-00014. PMID: 11468536.
- Kim H, Yi NJ, Lee J, Kim J, Moon MR, Jeong J, Lee JM, You TS, Suh SW, Park MS, Choi Y, Hong G, Lee HW, Lee KW, Suh KS. Safety of reduced dose of mycophenolate mofetil combined with tacrolimus in living-donor liver transplantation. Clin Mol Hepatol. 2014 Sep;20(3):291-9. doi: 10.3350/cmh.2014.20.3.291. Epub 2014 Sep 25. PMID: 25320733; PMCID: PMC4197178.
- Yau WP, Vathsala A, Lou HX, Chan E. Is a standard fixed dose of mycophenolate mofetil ideal for all patients? Nephrol Dial Transplant. 2007 Dec;22(12):3638-45. doi: 10.1093/ndt/gfm468. Epub 2007 Jul 19. PMID: 17640939.
- Oellerich M, Shipkova M, Schütz E, Wieland E, Weber L, Tönshoff B, Armstrong VW. Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit. 2000 Feb;22(1):20-6. doi: 10.1097/00007691-200002000-00004. Erratum in: Ther Drug Monit 2000 Aug;22(4):500. PMID: 10688252.
- Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T. Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit. 2001 Aug;23(4):305-15. doi: 10.1097/00007691-200108000-00001. PMID: 11477311.
- Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, Tönshoff B, Holt DW, Chapman J, Gelder Tv; Transplantation Society (TTS) Consensus Group on TDM of MPA. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 2010 Feb;5(2):341-58. doi: 10.2215/CJN.07111009. Epub 2010 Jan 7. PMID: 20056756.
- Yamada S, Shiohira H, Uehara H, Hokama N, Saitou S, Ooshiro Y. Implications of Clinical Mycophenolate Mofetil Dose According to Individual Body Weight in Japanese Renal Transplant Recipients. Transplant Proc. 2016 Jan-Feb;48(1):35-41. doi: 10.1016/j.transproceed.2015.11.014. PMID: 26915840.
- Khosroshahi HT, Shoja MM, Peyrovifar A, Hashemi SR, Amjadi M. Mycophenolate mofetil dose reduction in renal transplant recipients: a 5-year follow-up study. Transplant Proc. 2009 Sep;41(7):2797-9. doi: 10.1016/j.transproceed.2009.07.036. PMID: 19765438.
- Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, Mulgaonkar S, Meier-Kriesche HU, Patel D, Bloom RD. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009 Jul;9(7):1607-19. doi: 10.1111/j.1600-6143.2009.02668.x. Epub 2009 May 20. PMID: 19459794.
- Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, Parikh CR. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008 Sep;23(9):2995-3003. doi: 10.1093/ndt/gfn158. Epub 2008 Apr 11. PMID: 18408075; PMCID: PMC2727302.
- Al-Bahou AA, Webb AR, Bloxam M, Anger LJBowman, Baliga RS, Brueckner AJ. Outcomes Associated with Mycophenolate Weight-Based Dosing in Varying Immunologic Risk Kidney Transplant Recipients. Am J Transplant. 2020; 20 (suppl 3).
- Knoll GA, MacDonald I, Khan A, Van Walraven C. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003 Sep;14(9):2381-6. doi: 10.1097/01.asn.0000079616.71891.f5. PMID: 12937317.
- Dave V, Polkinghorne KR, Leong KG, Kanellis J, Mulley WR. Initial mycophenolate dose in tacrolimus treated renal transplant recipients, a cohort study comparing leukopaenia, rejection and long-term graft function. Sci Rep. 2020 Nov 9;10(1):19379. doi: 10.1038/s41598-020-76379-6. PMID: 33168923; PMCID: PMC7653942.
- Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009 Mar;24(3):1039-47. doi: 10.1093/ndt/gfn667. Epub 2008 Dec 22. PMID: 19103734.
- Humar A, Johnson EM, Payne WD, Wrenshall L, Sutherland DE, Najarian JS, Gillingham KJ, Matas AJ. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant. 1997 Dec;11(6):623-7. PMID: 9408697.
- Rodrigo E, Fernández-Fresnedo G, Ruiz JC, Piñera C, Palomar R, González-Cotorruelo J, Zubimendi JA, De Francisco AL, Sanz de Castro S, Arias M. Similar impact of slow and delayed graft function on renal allograft outcome and function. Transplant Proc. 2005 Apr;37(3):1431-2. doi: 10.1016/j.transproceed.2005.02.052. PMID: 15866627.
- Chapman TM, Keating GM. Basiliximab: a review of its use as induction therapy in renal transplantation. Drugs. 2003;63(24):2803-35. doi: 10.2165/00003495-200363240-00009. PMID: 14664658.
- Taber DJ, Egede LE, Baliga PK. Outcome disparities between African Americans and Caucasians in contemporary kidney transplant recipients. Am J Surg. 2017 Apr;213(4):666-672. doi: 10.1016/j.amjsurg.2016.11.024. Epub 2016 Nov 18. PMID: 27887677; PMCID: PMC5373991.
- Heilman RL, Reddy KS, Mazur MJ, Moss AA, Post DJ, Petrides S, Mulligan DC. Acute rejection risk in kidney transplant recipients on steroid-avoidance immunosuppression receiving induction with either antithymocyte globulin or basiliximab. Transplant Proc. 2006 Jun;38(5):1307-13. doi: 10.1016/j.transproceed.2006.02.116. PMID: 16797289.
- Woodle ES, Alloway RR, Buell JF, Alexander JW, Munda R, Roy-Chaudhury P, First MR, Cardi M, Trofe J. Multivariate analysis of risk factors for acute rejection in early corticosteroid cessation regimens under modern immunosuppression. Am J Transplant. 2005 Nov;5(11):2740-4. doi: 10.1111/j.1600-6143.2005.01090.x. PMID: 16212635.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996 Apr 15;61(7):1029-37. PMID: 8623181.
- Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. European Mycophenolate Mofetil Cooperative Study Group. Lancet. 1995 May 27;345(8961):1321-5. PMID: 7752752.
- Vanhove T, Kuypers D, Claes KJ, Evenepoel P, Meijers B, Naesens M, Vanrenterghem Y, Cornelis T, Bammens B. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013 Aug;26(8):813-21. doi: 10.1111/tri.12133. Epub 2013 Jun 10. PMID: 23746202.