More Information
Submitted: August 20, 2022 | Approved: September 13, 2022 | Published: September 14, 2022
How to cite this article: Obereisenbuchner F, Bader-Zollner S, Hans-Paul S. Administration of G-CSF for PBSC collection may unmask pre-existing IgA-nephropathy: A case report. J Clini Nephrol. 2022; 6: 079-082.
DOI: 10.29328/journal.jcn.1001094
Copyright License: © 2022 Obereisenbuchner F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: IgA nephropathy; G-CSF; Hematopoietic stem cell donation; Disease exacerbation
Administration of G-CSF for PBSC collection may unmask pre-existing IgA-nephropathy: A case report
Florian Obereisenbuchner1,2*, Sabine Bader-Zollner3 and Hans-Paul Schobel2
1Faculty of Medicine, LMU Munich, Germany
2Department of Internal Medicine-Nephrology, Hospital Starnberg, Germany
3Nephrocare Dialysis Unit, Starnberg, Germany
*Address for Correspondence: Florian Obereisenbuchner, Faculty of Medicine, LMU Munich, Germany, Email: [email protected]
It is utterly important to ensure the safety of stem cell donors and limit the incidence of long-term adverse events. Additionally, the willingness to donate the potentially life-saving stem cells, depends among other reasons, on the donor’s trust in the safety of the procedure as our case highlights. Here we present the case of a 35-year-old patient who developed macrohematuria and proteinuria following peripheral blood stem cell (PBSC) donation. 4 years later he was diagnosed with IgA-nephropathy (IgAN) and the disorder was causally attributed to the PBSC donation. He discouraged his family and friends from registering as donors because of this. In the current case report, we review the literature on the relationship between IgAN and PBSC donation and suggest under which conditions stem cell donation can still be performed even with a prior diagnosis of IgAN.
A 35-year-old patient with microhematuria, proteinuria and arterial hypertension was referred to a nephrologist in an ambulatory care setting. These were accidental findings in an otherwise symptom-free patient during a check-up at the patient’s general practitioner. When presenting to the nephrologist, the patient reported no specific symptoms, especially no foamy urine, dysuria, or infection during the past weeks. More in-depth history taking brought up that his mother complains of hematuria since childhood and experienced a nephritis of unknown etiology at the age of 30. His grandfather had Diabetes mellitus and unclear kidney disease which might have been related to his Diabetes. His greatuncle also had an unclear kidney disease requiring dialysis and later on a kidney transplant. The patient himself reported a first episode of microhematuria 20 years ago during a routine check-up for military service that was not followed up in the absence of other symptoms. Following a peripheral blood stem cell donation (PBSC) 4 years ago he experienced an episode of macrohematuria and reported repeated episodes of dark urine throughout the last years. His daily urine excretion now matched his daily intake of liquids.
The physical examination (heart, lungs and abdomen without pathological findings, no edema, no infect signs) of the 1.90 m tall patient, with a body weight of 107 kg, was unremarkable except for an elevated blood pressure of 156/102 mmHg.
Urine examination revealed 100 mg/dl proteins with albuminuria and 200 erythrocytes per µl. No dysmorphic erythrocytes or leukocytes were observed, and the rest was unremarkable with a pH of 6.0. Blood examination was unremarkable with normal electrolytes and balanced acid-bases status. Serologic testing for ANA, p- and c-ANCA and anti-GBM was negative. The complement factors C3-, C4-, serum IgA-level and serum-protein electrophoresis were normal.
The patient was prescribed Candesartan 4 mg bid and referred to our hospital to perform a renal biopsy as he was assumed to have signs of an IgA-nephropathy (IgAN).
At admission to the hospital, the patient was in good general condition, he had no complaints and reported no history of underlying chronic disease. Vital signs at admission were normal (heart rate 61/min, body temperature 36.5 °C, oxygen saturation 99% in ambient air, respiratory frequency 16/min) except for an increased blood pressure of 149/84 mmHg. There was no edema or signs of infection.
The ECG was normal. The kidney function parameters were normal: serum-creatinine 1.02 mg/dl with an eGFR (CKD-EPI formula) of 95 ml/min/1.73m² as were the serum-electrolytes. His urine showed 200 erythrocytes per microliter, 100 mg/dl proteins, and a pH of 6.0. There were no leucocytes, no nitrite, no glucose and no ketone bodies detectable.
A spot-urine quantification of the proteinuria revealed total proteinuria of 755 mg/l and a urinary protein to creatinine ratio of 515 mg/g (normal range: < 100 mg/g).
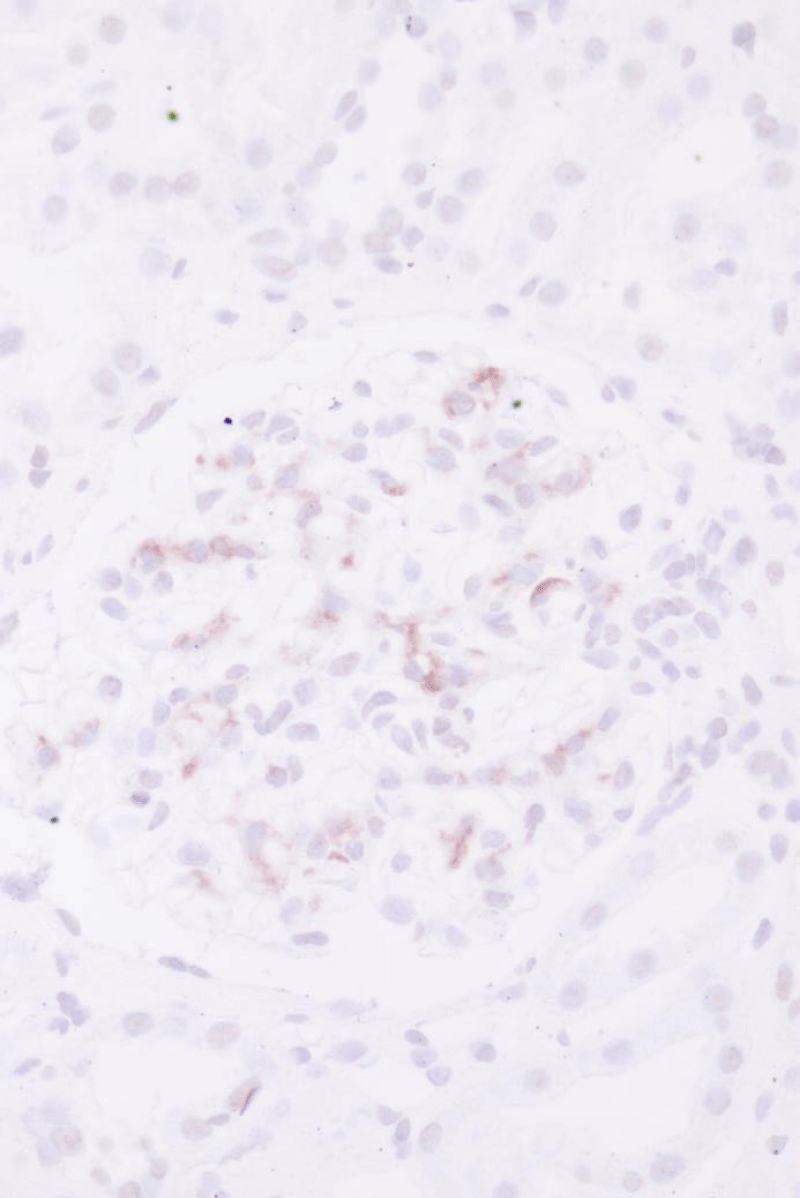
The kidney biopsy (Figures 1-3) showed an early IgAN with an Oxford classification of M0 E0 S0 T0 [1]. We continued the therapy with Candesartan 4 mg bid. The patient regularly visits his nephrologist (SBZ) for clinical monitoring and control of proteinuria and kidney function. Until now (mid-2022), the proteinuria and kidney function has been stable and the blood pressure is within the normal range.
Figure 1: Light microscopy with H.E. staining demonstrates a glomerulus with mesangial matrix- and cell proliferation.
Figure 2: Immunohistochemistry showing glomerular IgA-deposits (l.)
Figure 3: Immunohistochemistry showing glomerular C3d deposits (r.).
During the history taking before admission, the patient stated that he first observed macroscopic hematuria a few days after he donated hematopoietic stem cells (peripheral blood stem cells = PBSC) four years ago and therefore assumed that his current renal condition was related to the PBSC. As a consequence, he advised his friends and family to avoid stem cell donations in general.
Is this justified?
Although the risks for the donors are generally considered to be very low [2,3], the theoretical possibility of exacerbation or manifestation of an underlying autoimmune or autoinflammatory disorder seemed plausible given the immune modulating effects of granulocyte colony-stimulating factor, used to mobilize bone marrow hematopoietic progenitor cells to prepare the leukapheresis. We, therefore, searched the medical literature concerning adverse events related to the administration of G-CSF. G-CSF is a naturally-occurring cytokine that interacts with many different cell types involved in immune responses and beyond [4]. Infections can trigger a 10-30fold increase in serum levels [5] while receiving a single 300 µg subcutaneous injection, the order of magnitude commonly used in the preparation for leukapheresis (~10 µg/kg body weight), leads to a 500-1000fold increase, which returns to normal levels after 48 h [6]. Exogenous G-CSF has both pro- and anti-inflammatory effects via the main cytokine pathways (for a review see [7]). The most common symptoms experienced by patients and healthy donors treated with G-CSF are bone pain (52% - 84%), followed by fatigue, fever, mild headaches, myalgia and arthralgia [8-11]. Transient spleen enlargement is observed in almost all patients [12,13] with rare cases of atraumatic splenic rupture. Most severe adverse events (except for allergic reactions) like flares of underlying autoimmune conditions, haematological malignancies and vascular complications are only reported in anecdotal reports or small case series and the causal involvement of G-CSF is not definitively established [8,14].
We further looked specifically for earlier reported cases of an exacerbation or manifestation of IgAN or other renal diseases following the administration of G-CSF. Our literature research yielded two cases of biopsy-proven newly diagnosed IgAN, two possible cases of IgAN in which it was obtained from a biopsy because of clinical remission as well as one exacerbation of a prior known, biopsy-proven IgAN [15-18]. Furthermore, one case of a progression from micro- to macrohematuria was observed in a register of 7850 PBSC donors. The macrohematuria resolved spontaneously after 6 weeks and returned to the prior benign microscopic haematuria, therefore no biopsy was performed [3]. We furthermore identified a case of focal segmental glomerulonephritis in a previously healthy donor [10] and a case of relapsing membranoproliferative glomerulonephritis following G-CSF co-administration in the course of chemotherapy for breast cancer [5]. Both patients showed no histopathological features of IgAN. The patterns were more suggestive of a vasculitis etiology. An older study addressing long-term risks of regular G-CSF administration in severe congenital neutropenia (SCN) patients reported a few patients with episodically recurring episodes of hematuria in the absence of underlying renal disease [19].
As underlying mechanisms by which G-CFS application might induce or aggravate kidney disease, especially IgA-nephropathy, the literature there are discussed the two following scenarios: First, G-CSF recruits neutrophils to immune complex deposits leading to an exacerbation of underlying or subclinical immune complex disease [11]. Second, G-CSF might injure the endothelium thereby leading to hematuria and/or proteinuria in susceptible individuals [4,20].
Taken together, given the current scientific evidence, neither the effect of G-CSF on hematuria and IgAN nor a putative mechanism of action is well understood.
Considering the general risk-benefit profile of bone marrow- vs. PBSC for stem cell acquisition, PBSC must still be considered advantageous in most cases (see e.g. [3,21]), especially given the fact that the reported adverse effects of G-CSF are only relatively mild and of transient nature.
According to our patient’s history, it must be assumed that his kidney disease was pre-existent and G-CSF administration for PBSC, although triggering a microhematuria episode, did not aggravate it. This is shown by the kidney biopsy results with a very low MEST-score, the presence of only mild to moderate proteinuria (< 1 g/g Creatinine) and his normal GFR 4 years after PBSC. However, a longer follow-up period and further case series or studies are needed to ensure that the prognosis of patients with IgAN or with a positive family history for IgAN or other immunological renal disorders is not negatively affected by the treatment with G-CSF.
This has several implications for future PBSC donors: the screening should include history taking for past episodes of hematuria and family history, an urine screening for hematuria and/or proteinuria and an evaluation of the kidney function (serum Creatinine/eGFR) to identify individuals with pre-existing but not yet diagnosed kidney disorders and at-risk individuals. If those tests show conspicuous results, a nephrologist should be consulted. Furthermore, positive screening results should prompt the informing of the potential donor that a possible underlying renal disease might be aggravated by the administration of G-CSF (although it is highly unlikely that renal disease is actually caused by it). Further research exploring the effects of G-CSF on healthy individuals as well as on patients with underlying immunological and/or autoimmune disorders (renal and non-renal) will be needed to better explain this rare but significant observation. Based on a 2015 consensus statement [22], potential donors with impaired kidney function should be advised to prefer bone marrow to PBSC donation, given the momentary uncertainty of potential risk for the individual donor.
Given the overall benign course of reported cases in literature as well as our own case, underlying IgA-nephropathy must not generally be considered a contraindication for PBSC donation. We strongly recommend, however, the involvement of a nephrologist and close monitoring of the potential donor, including follow-up after the end of PBSC donation.
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009 Sep;76(5):534-45. doi: 10.1038/ki.2009.243. Epub 2009 Jul 1. PMID: 19571791.
- Halter J, Kodera Y, Ispizua AU, Greinix HT, Schmitz N, Favre G, Baldomero H, Niederwieser D, Apperley JF, Gratwohl A. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica. 2009 Jan;94(1):94-101. doi: 10.3324/haematol.13668. Epub 2008 Dec 4. PMID: 19059940; PMCID: PMC2625420.
- Miller JP, Perry EH, Price TH, Bolan CD Jr, Karanes C, Boyd TM, Chitphakdithai P, King RJ. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008 Sep;14(9 Suppl):29-36. doi: 10.1016/j.bbmt.2008.05.018. PMID: 18721778.
- Anderlini P, Champlin RE. Biologic and molecular effects of granulocyte colony-stimulating factor in healthy individuals: recent findings and current challenges. Blood. 2008 Feb 15;111(4):1767-72. doi: 10.1182/blood-2007-07-097543. Epub 2007 Dec 5. PMID: 18057230.
- Arora S, Bhargava A, Jasnosz K, Clark B. Relapsing acute kidney injury associated with pegfilgrastim. Case Rep Nephrol Urol. 2012 Jul;2(2):165-71. doi: 10.1159/000345278. Epub 2012 Nov 21. PMID: 23326257; PMCID: PMC3542938.
- de Haas M, Kerst JM, van der Schoot CE, Calafat J, Hack CE, Nuijens JH, Roos D, van Oers RH, von dem Borne AE. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on circulating neutrophils. Blood. 1994 Dec 1;84(11):3885-94. PMID: 7524751.
- Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood. 1999 Jan 15;93(2):425-39. PMID: 9885204.
- Tigue CC, McKoy JM, Evens AM, Trifilio SM, Tallman MS, Bennett CL. Granulocyte-colony stimulating factor administration to healthy individuals and persons with chronic neutropenia or cancer: an overview of safety considerations from the Research on Adverse Drug Events and Reports project. Bone Marrow Transplant. 2007 Aug;40(3):185-92. doi: 10.1038/sj.bmt.1705722. Epub 2007 Jun 11. PMID: 17563736.
- Dale DC, Cottle TE, Fier CJ, Bolyard AA, Bonilla MA, Boxer LA, Cham B, Freedman MH, Kannourakis G, Kinsey SE, Davis R, Scarlata D, Schwinzer B, Zeidler C, Welte K. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol. 2003 Feb;72(2):82-93. doi: 10.1002/ajh.10255. PMID: 12555210.
- Nasilowska-Adamska B, Perkowska-Ptasinska A, Tomaszewska A, Serwacka A, Marianska B. Acute glomerulonephritis in a donor as a side effect of allogeneic peripheral blood stem cell mobilization with granulocyte colony-stimulating factor. Int J Hematol. 2010 Dec;92(5):765-8. doi: 10.1007/s12185-010-0730-6. Epub 2010 Dec 1. PMID: 21120643.
- Cottle TE, Fier CJ, Donadieu J, Kinsey SE. Risk and benefit of treatment of severe chronic neutropenia with granulocyte colony-stimulating factor. Semin Hematol. 2002 Apr;39(2):134-40. doi: 10.1053/shem.2002.31914. PMID: 11957197.
- Stroncek D, Shawker T, Follmann D, Leitman SF. G-CSF-induced spleen size changes in peripheral blood progenitor cell donors. Transfusion. 2003 May;43(5):609-13. doi: 10.1046/j.1537-2995.2003.00384.x. PMID: 12702182.
- Stroncek DF, Dittmar K, Shawker T, Heatherman A, Leitman SF. Transient spleen enlargement in peripheral blood progenitor cell donors given G-CSF. J Transl Med. 2004 Jul 21;2(1):25. doi: 10.1186/1479-5876-2-25. PMID: 15268759; PMCID: PMC493285.
- Bennett CL, Evens AM, Andritsos LA, Balasubramanian L, Mai M, Fisher MJ, Kuzel TM, Angelotta C, McKoy JM, Vose JM, Bierman PJ, Kuter DJ, Trifilio SM, Devine SM, Tallman MS. Haematological malignancies developing in previously healthy individuals who received haematopoietic growth factors: report from the Research on Adverse Drug Events and Reports (RADAR) project. Br J Haematol. 2006 Dec;135(5):642-50. doi: 10.1111/j.1365-2141.2006.06312.x. Epub 2006 Oct 19. PMID: 17054431.
- Lee JB, Billen A, Lown RN, Potter MN, Craddock CF, de Lavallade H, Shaw BE, Sharpe CC. Exacerbation of IgA nephropathy following G-CSF administration for PBSC collection: suggestions for better donor screening. Bone Marrow Transplant. 2016 Feb;51(2):286-7. doi: 10.1038/bmt.2015.224. Epub 2015 Oct 5. PMID: 26437069.
- Nampoothiri RV, Kumar V, Bharati J, Lad S, Arora K, Malhotra P, Lad D. Hematopoietic stem cell donor with IgA nephropathy: Challenges and management algorithm. Transfus Apher Sci. 2020 Aug;59(4):102781. doi: 10.1016/j.transci.2020.102781. Epub 2020 May 8. PMID: 32409153.
- Funakoshi Y, Nazneen A, Nakashima Y, Nakashima K, Okada M, Taguchi T, Moriuchi H. Possible involvement of G-CSF in IgA nephropathy developing in an allogeneic peripheral blood SCT donor. Bone Marrow Transplant. 2010 Sep;45(9):1477-8. doi: 10.1038/bmt.2010.4. Epub 2010 Mar 1. PMID: 20190844.
- Billen A, Lee JBL, Lown R, Potter M, Craddock C, de Lavallade H, et al. A Proven and Probable Case of Pre-Existing IgA Nephropathy Exacerbated By Administration of G-CSF. Biology of Blood and Marrow Transplantation. 2015; 21(2):S272-S3. doi: 10.1016/j.bbmt.2014.11.432.
- Bonilla MA, Dale D, Zeidler C, Last L, Reiter A, Ruggeiro M, Davis M, Koci B, Hammond W, Gillio A, Welte K. Long-term safety of treatment with recombinant human granulocyte colony-stimulating factor (r-metHuG-CSF) in patients with severe congenital neutropenias. Br J Haematol. 1994 Dec;88(4):723-30. doi: 10.1111/j.1365-2141.1994.tb05110.x. PMID: 7529539.
- Falanga A, Marchetti M, Evangelista V, Manarini S, Oldani E, Giovanelli S, Galbusera M, Cerletti C, Barbui T. Neutrophil activation and hemostatic changes in healthy donors receiving granulocyte colony-stimulating factor. Blood. 1999 Apr 15;93(8):2506-14. PMID: 10194429.
- Burns LJ, Logan BR, Chitphakdithai P, Miller JP, Drexler R, Spellman S, Switzer GE, Wingard JR, Anasetti C, Confer DL; Blood and Marrow Transplant Clinical Trials Network. Recovery of Unrelated Donors of Peripheral Blood Stem Cells versus Recovery of Unrelated Donors of Bone Marrow: A Prespecified Analysis from the Phase III Blood and Marrow Transplant Clinical Trials Network Protocol 0201. Biol Blood Marrow Transplant. 2016 Jun;22(6):1108-1116. doi: 10.1016/j.bbmt.2016.02.018. Epub 2016 Mar 21. PMID: 27013014; PMCID: PMC4867293.
- Worel N, Buser A, Greinix HT, Hägglund H, Navarro W, Pulsipher MA, Nicoloso de Faveri G, Bengtsson M, Billen A, Espino G, Fechter M, Giudice V, Hölig K, Kanamori H, Kodera Y, Leitner G, Netelenbos T, Niederwieser D, van Walraven SM, Rocha V, Torosian T, Vergueiro C, Weisdorf D, Yabe H, Halter JP. Suitability Criteria for Adult Related Donors: A Consensus Statement from the Worldwide Network for Blood and Marrow Transplantation Standing Committee on Donor Issues. Biol Blood Marrow Transplant. 2015 Dec;21(12):2052-2060. doi: 10.1016/j.bbmt.2015.08.009. Epub 2015 Aug 10. PMID: 26271194.