More Information
Submitted: May 21, 2022 | Approved: June 15, 2022 | Published: June 16, 2022
How to cite this article: Mambap TA, Toukam NAC, Maimouna M, Teuwafeu DG, Ashuntantang EG. Incidence, risk factors, and outcomes of acute kidney injury among hiv positive medical admissions at the Bamenda Regional Hospital. J Clini Nephrol. 2022; 6: 068-073.
DOI: 10.29328/journal.jcn.1001092
Copyright License: © 2022 Mambap TA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Acute kidney injury; HIV; Medical admissions; Incidence; Risk factors; Outcomes; Cameroon
Incidence, risk factors, and outcomes of acute kidney injury among hiv positive medical admissions at the Bamenda Regional Hospital
Mambap Tatang Alex1,2*, Toukam Nguebmegne Arielle Carelle1, Mahamat Maimouna3, Teuwafeu Denis Georges4 and Ashuntantang Gloria Enow3
1Department of Clinical Sciences, Faculty of Health Sciences, The University of Bamenda, Cameroon
2Bamenda Regional Hospital, Cameroon
3Department of Internal Medicine and Specialties, Faculty of Medicine and Biomedical Sciences, The University of Yaounde I, Cameroon
4Department of Internal Medicine and Pediatrics, Faculty of Health Sciences, The University of Buea, Cameroon
*Address for Correspondence: Mambap Tatang Alex, Department of Clinical Sciences, Faculty of Health Sciences, The University of Bamenda, Cameroon, Email: [email protected]
Background: There is a paucity of data on the burden of acute kidney injury (AKI) in hospitalized HIV-infected patients in Sub-Saharan Africa in the “test and treat” era.
Objectives: To study the incidence, risk factors, and outcomes of AKI among HIV-positive medical admissions in a secondary hospital.
Materials and methods: We prospectively screened adult HIV-positive patients who gave their informed consent and were admitted to the Bamenda Regional Hospital for AKI from February to June 2020. We excluded participants with Chronic Kidney Disease (CKD) Stage 5 and those with confounders of serum creatinine. On admission and after 2-7 days, we extracted a venous blood sample from each participant to evaluate serum creatinine and diagnose AKI. The participants were then followed up on until they were discharged or died. We measured the need for dialysis, access to dialysis, and renal recovery at three months for patients with AKI. The amended KDIGO 2012 criteria were used to define and classify AKI. The University of Bamenda’s institutional review board provided ethical approval.
Results: A total of 206 participants (39.8% men) were enrolled, with a mean (SD) age of 45.71(13.13) years. On enrolment, 89.8% (n = 185) of the participants were on combination antiretroviral therapy (c-ART), with 81.6% (n = 151) on tenofovir-containing regimens. The WHO HIV clinical stages 3 and 4 were present in 81.5% (n = 168) of the individuals. The most common reason for hospitalization was opportunistic infections (69.8%; n = 142). AKI was found in 30.6% (n = 63) of the patients, with 58.7% (n = 37) of them being classified as KDIGO stage 3.
A total of 12 (42.9%) participants out of the 28 in need, were dialyzed. AKI was independently associated with use of traditional medicines (aOR = 2.9; 95% CI 1.4-6.3; p = 0.006), WHO HIV stages 3 and 4 (aOR = 4.1; 95% CI 1.1-15.7; p = 0.038), hypotension (aOR = 3.3; 95% CI 1.4-7.8; p = 0.008) and low haemoglobin level ≤ 8.0 g/dl (aOR = 3.5; 95% CI 1.7-7.4; p = 0.001). The AKI group used to have a significantly higher mortality rate (42.9% vs. 16.1%; p < 0.001). Renal recovery was complete in 66.7% of the 30 survivors at three months, partial in 13.3%, and no recovery in 20% of the survivors.
Conclusion: Despite the growing use of combination antiretroviral medication, significant immunosuppression is still common in hospitalized HIV-positive patients, increasing the risk of AKI and worsening prognosis. In this high-risk population, early detection of AKI with renal function monitoring may improve results.
The human immune deficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) pandemic has caused far-reaching effects in low-income countries, especially sub-Saharan Africa. The introduction and widespread use of combination antiretroviral therapy (c-ART) and other precautionary and therapeutic strategies have greatly improved the prognosis and quality of life of HIV-positive people, with a significant reduction in HIV-related mortality and morbidity [1-3]. Renal dysfunction, especially acute kidney injury (AKI), is an important cause of hospitalization and mortality in HIV‑positive patients, despite increased survival thanks to c-ART [4-6]. The majority of investigations on AKI in HIV-infected patients have been observational studies conducted in nephrology, infectious disease units, and intensive care units, particularly in high-income countries, with referred patients or critically ill patients. However, in non-renal medical admissions, particularly in low-income countries where renal function monitoring is not routine and complications cannot be properly assessed, the true incidence of AKI may be higher and outcomes poorer due to late presentation to the hospital, a poor socioeconomic profile, and poor healthcare personnel quality. The goal of this study was to assess the prevalence, risk factors, and outcomes of AKI among HIV-positive medical admissions in a government-funded semi-urban hospital with subsidised conventional hemodialysis.
Study setting
The Bamenda regional hospital is located in the capital of the North-West region and serves as the main reference hospital of the region with an estimated population of around 1-5 million. It has 180 beds, a central laboratory, and standard radiology services. There are internal medicine, pediatric, surgical, and gynaeco-obstetrical services with both in and outpatient clinics. It also has the unique nephrology, and hemodialysis as well as the HIV reference center of the region. During the study period, HIV viral load and CD4 assay were not available in the region.
tudy design
This was a prospective study including all adult HIV-positive patients who gave their informed consent and were admitted to the medical wards of the Bamenda regional hospital from February to June 2020. We measured serum creatinine on admission and again between days 2 and 7 to diagnose AKI in each consenting patient. A serum creatinine assay was performed on discharge and 90 days following diagnosis for patients with AKI. A semi-automated biochemical analyzer was used to quantify serum creatinine using the Jaffe kinetic technique (MINDRAY BIOSMART 240). The following variables were also gathered: Clinical data, such as co-morbidities, HIV-related characteristics, primary diagnosis, and clinical parameters; socio-demographic information, such as date of birth, sex, level of education, and source of funding. The study outcomes were the incidence of AKI, the stages, the need for dialysis, the access to dialysis, the patient mortality, and the renal recovery 90 days after diagnosis.
Definition of operational terms
AKI was defined according to the modified KDIGO 2012 criteria [7] as an increase or decrease in serum creatinine of 0.3 mg/dl or greater (≥ 26.5 µmol/l) within 48 hours; or an increase in serum creatinine to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days. AKI severity was graded using the KDIGO 2012 criteria [8]. HIV infection was based on a self-reported or a documented medical history of HIV, ongoing drug treatments, or a positive serologic test for HIV, and was staged according to the WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children [9]. If individuals were diagnosed with AKI on arrival to the hospital, it was labeled community-acquired AKI. Renal recovery was considered as complete when serum creatinine was equal to or lower than the baseline or reference value at discharge or after 90 days. Renal recovery was partial if serum creatinine fell below the diagnosis value but not to baseline or reference, and no recovery if serum creatinine did not fall or the patient remained on dialysis. The nephrologist was in charge of diagnosing AKI and its causes.
Ethical approval was obtained from the ethical committee board of the University of Bamenda (ID Number: 2020/0006H/UBa/IRB) and administrative authorization from the director of the Bamenda regional hospital.
Statistical analysis
Data collected were analyzed using the software statistical package for social sciences (SPSS) version 26.0. Continuous variables were presented as mean ± standard deviation and/or as median (inter-quartile range). Categorical variables were expressed as percentages. Both univariate and multivariate analysis was performed with both logistic regression to identify the potential risk factors of AKI. A p - value < 0.05 was considered significant.
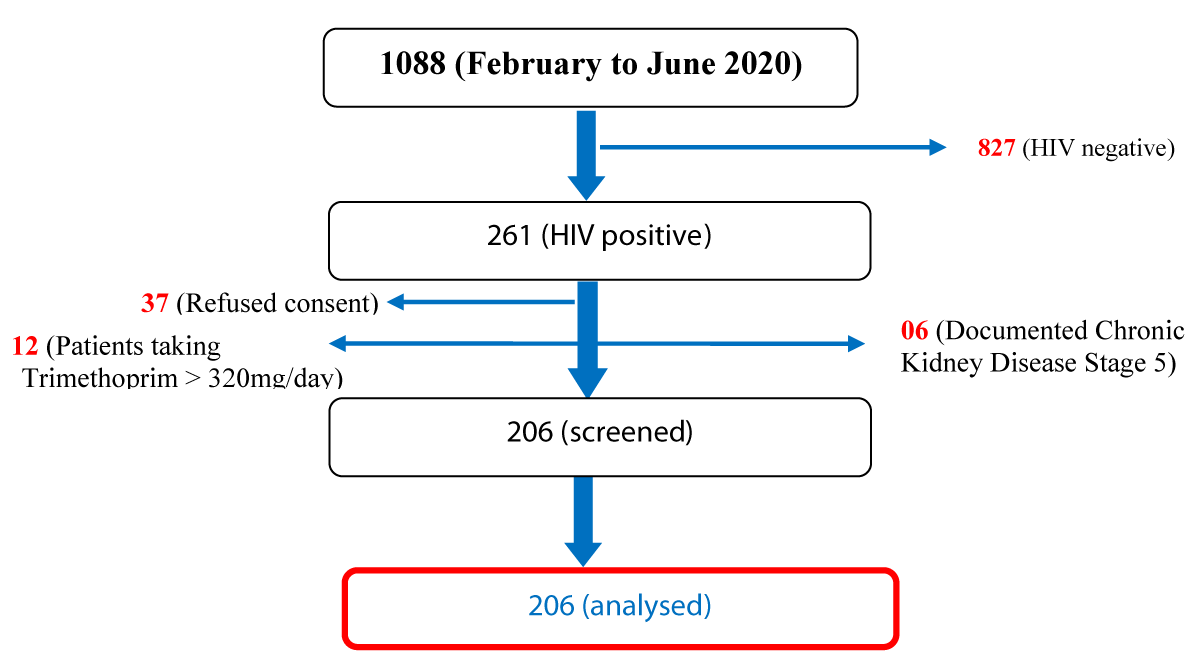
During the study period, 261 of the 1088 medical admissions at the Bamenda Regional Hospital were HIV positive, representing a proportion of 23.99%. Patients lacking a second serum creatinine value (withdrawal of permission, discharge against medical advice, and death), patients with Chronic Kidney Disease Stage 5, and individuals on high dose Bactrim (>1600 mg of Trimethoprim/24 hours) were all removed, leaving us with 55 patients. Males made up 39.8% (n = 82) of the 206 participants, while females made up 60.2% (n = 124). The average (SD) age was 45.71 (13.13) years (range: 18-84 years), and more than a third of them were unemployed (n = 77) (Figure 1).
Figure 1: Flow chart showing participant’s recruitment.
Obesity (35.9%; n = 74), hypertension (22.3%; n = 46), and diabetes mellitus (8.3%; n = 17) were the most frequent comorbid conditions (see Table 1). Opportunistic affections (69.8%; n = 142) consisted over two thirds of the reasons for admissions with community acquired pneumonia (19.0%; n = 27), tuberculosis (18.3%; n = 26), and chronic diarrhoea (12.7%; n = 18), being the most frequent diseases. Severe malaria (12.5%; n = 8) and congestive heart failure (10.9%; n = 7) were the most frequent non-opportunistic diseases (Table 1). Trimethoprim (n = 134, 65.0%), fluconazole (n = 65, 31.6%), traditional medicines (n = 52, 25.2%), and non-steroidal anti-inflammatory drugs (n = 52, 25.2%) were the most frequent drugs in use. In all, 89.8% (n = 185) were on combined antiretroviral therapy (c-ART) at the time of enrolment, among which only 37.8% (n = 70) were compliant to treatment, and 81.6% (n = 151) were on Tenofovir-containing regimens (Table 1). Over 80% of the participants were in WHO Clinical Stage 3 and 4 (81.5%; n = 168), and about three-quarter had anaemia (71.8%; n = 148), with the mean (SD) haemoglobin level being 9.3 (2.7) g/dl.
| Table 1: Socio-demographic and clinical characteristics of the study population. | |
| Clinical characteristics (N = 206) | Total n (%) |
| Mean age (SD) (in years) | 45.71 (13.13) |
| Sex, Female | 124 (60.2%) |
| Level of education | |
| < secondary education | 58 (28.2) |
| ≥ secondary education | 148 (71.8) |
| Employment status | |
| Unemployed | 77 (37.4%) |
| Public Sector | 16 (7.8%) |
| Private sector | 48 (23.3%) |
| Informal Sector | 54 (26.2%) |
| Retired | 11 (5.3%) |
| Source of funding | |
| Self | 48 (23.3%) |
| Family | 150 (72.8%) |
| Insurance companies | 2 (1.0%) |
| Hospital social services | 6 (2.9%) |
| Co-morbidities | |
| Overweight/ Obesity | 74 (35.9) |
| Hypertension | 46 (22.3) |
| Diabetes | 17 (8.3) |
| Current drug use | |
| Co-trimoxazole | 134 (65.0) |
| Fluconazole | 65 (31.6) |
| Traditional medicines | 52 (25.2) |
| Admission diagnosis | |
| Opportunistic infections (n = 142; 68.9%) | |
| Community acquired pneumonia | 27 (19.0) |
| Tuberculosis | 26 (18.3) |
| Chronic diarrhoea | 18 (12.7) |
| Non Opportunistic infections (n= 64; 31.1%) | |
| Severe Malaria | 8 (12.5) |
| Congestive Heart Failure | 7 (10.9) |
| DVT / Arterial limb Ischemia | 7 (10.9) |
| HIV characteristics (n = 206) | |
| Stage 3 | 109 (52.9) |
| Stage 4 | 59 (28.6) |
| Absolute Lymphocyte Count (in cells/mm3) <1000 | 112 (54.4) |
| On cART | 185 (89.8) |
| Tenofovir-containing regimen (n = 185) | 151 (81.6) |
| Adherence to cART (n = 185) | 70 (37.8) |
| Means Baseline serum creatinine (SD) (in mg/dl) | 0.96 (0.80-1.24) |
| HIV: human immune deficiency virus; cART: combined antiretroviral therapy; DVT: Deep Venous Thrombosis; SD: Standard deviation | |
Of the 206 participants, 63 had AKI, thus an incidence of 30.6%. Of the 63 participants with AKI, 92.1% (n = 58) were community-acquired and more than half (58.7%; n = 37) were at KDIGO stage 3. Among those who developed AKI, 68.3% (n = 43) had renal AKI, with acute tubular necrosis (39.7%; n = 25) related to sepsis and nephrotoxic medications being the most common etiology (Table 2).
| Table 2: Nature, mechanism, severity, and etiology of AKI amongst participants. | |
| Type and severity | Total n (%) |
| Type of AKI (n = 63) | |
| Community-acquired AKI | 58 (92.1) |
| Hospital-acquired AKI | 5 (7.9) |
| Severity of AKI (n = 63) | |
| Stage 1 | 15 (23.8) |
| Stage 2 | 11 (17.5) |
| Stage 3 | 37 (58.7) |
| Mechanism and Etiology (n = 63) | |
| Pre-renal AKI | (n = 18; 28.6%) |
| Renal AKI | (n = 43; 68.3%) |
| Acute Tubular Necrosis | 25 (39.7) |
| Sepsis | 17 (27.0) |
| Toxic | 8 (12.7) |
| Acute Glomerulonephritis | 8 (12.7) |
| Malignant Hypertension | 6 (9.5) |
| Acute Interstitial Nephritis | 4 (6.3) |
| Post-renal AKI | (n = 2; 3.2%) |
After adjusting for other factors, AKI was found to be associated with the current use of traditional medicines (aOR = 2.927; 95% CI = 1.367-6.270; p = 0.006), HIV stages 3 and 4 (aOR = 4.122; 95% CI = 1.081-15.720; p = 0.038), hypotension (aOR = 3.252; 95% CI = 1.360-7.776; p = 0.008), and low hemoglobin level ≤ 8.0g/dl (aOR = 3.515; 95% CI = 1.673-7.385; p = 0.001) (Table 3). Twenty-eight (44.4%) of the 63 patients with AKI needed dialysis, and 12 (42.9%) of them had access to it. Dialysis indications and causes for dialysis inaccessibility are shown in Table 4.
| Table 3: Factors associated with AKI on Multivariate analysis. | |||
| Variables | aOR | 95% CI | p-value |
| Hypertension | 2.55 | 0.83 - 7.77 | 0.101 |
| ACEIs | 1.13 | 0.27 - 4.67 | 0.871 |
| NSAIDs | 1.54 | 0.70 - 3.37 | 0.286 |
| Traditional medicines | 2.93 | 1.37 - 6.27 | 0.006 |
| WHO Clinical Staging of HIV Stage 3 and 4 | 4.12 | 1.08 - 15.72 | 0.038 |
| Absolute Lymphocyte Count (in cells/mm3) <1000 | 1.21 | 0.59 - 2.47 | 0.597 |
| Systolic blood pressure (mmHg) ≤90mmHg | 3.25 | 1.36 - 7.78 | 0.008 |
| Fever | 0.46 | 0.20 - 1.04 | 0.062 |
| Haemoglobin level (g/dl) ≤8.0 | 3.52 | 1.67 - 7.39 | 0.001 |
| Hospital stay (in days) ≥12 | 1.23 | 0.60 - 2.51 | 0.575 |
| aOR: adjusted Odds Ratio; ACEIs: Angiotensin-converting enzyme inhibitors; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; WHO: World Health Organization | |||
| Table 4: Indications for dialysis, reasons for non-access to dialysis, and renal recovery. | ||
| Items | Frequency (n) | Percentage (%) |
| Indications for dialysis (n = 28) | ||
| Overt uremic manifestations | 13 | 46.5 |
| Severe Hyperkalaemia | 9 | 32.1 |
| Refractory Fluid Overload | 3 | 10.7 |
| Oliguria/Anuria > 12hrs | 2 | 7.1 |
| Severe metabolic acidosis | 1 | 3.6 |
| Reason for non-access to dialysis (n = 16) | ||
| Early death | 7 | 43.8 |
| Lack of funds | 6 | 37.5 |
| Lack of materials | 2 | 12.5 |
| Left against medical advice | 1 | 6.3 |
| Renal recovery at 3 months (n = 30) | ||
| Complete recovery | 20 | 66.7 |
| Partial recovery | 4 | 13.3 |
| No recovery | 6 | 20.0 |
Of the 206 participants, 50 died during hospitalization giving an in-hospital mortality rate of 24.3% (50/206), with the rate significantly higher in the AKI group compared to the non-AKI group (42.9% vs. 16.1%; p < 0.001). The median length of hospital stay was 9.0 (6.0-15.0) days.
Of participants with AKI (63/206), 27(42.8%) died in hospital, and 6 (16.6%) left against medical advice. Of the remaining 30 participants after 3 months, 66.7% (20/30) had a complete renal recovery, 13.3% (4/30) had partial recovery, and 20.0% (6/30) had no recovery of renal function (Table 4).
In this cohort study of HIV-infected patients with 89.8% on c-ART, we observed a 30.6% incidence of AKI, with 58.7% in KDIGO stage 3, with a dialysis access rate of 42.9% (12/28). Current use of traditional medicines (aOR = 2.927; p = 0.006), HIV stage 3 and 4 (aOR = 4.122; p = 0.038), hypotension (aOR = 3.252; p = 0.008) and low haemoglobin level ≤ 8.0 g/dl (aOR = 3.515; p = 0.001) were independently associated with AKI. Mortality was significantly higher in the AKI group compared to the non-AKI group (42.9% vs. 16.1%; p < 0.001) and renal recovery at 3 months was complete in 66.7% of survivors.
The reported incidence of AKI in HIV-infected patients varies from 5.7% to 66% [4,10-20], depending on the study setting, study design, definition of AKI used, and characteristics of the study population. It is highest among critically ill patients, and lowest among ambulatory patients. In our study, we observed a high 30.6% incidence of AKI, which falls within that range. However, this finding is in contrast to the reported pooled worldwide incidence of AKI of 21.6% [21]. Lower AKI incidence rates of 5.7%-19.6% were reported amongst HIV-positive medical admissions in many studies [4,10,13-16,18,19]. Our high incidence rates can be explained by a number of factors. Not only did we screen all HIV-positive patients admitted for AKI, but we also found that two-thirds of the population had opportunistic infections, which are a primary cause of AKI in HIV-infected patients [7,22,23]. Our findings are in line with those of previous research in the same nation, which revealed incidence rates of AKI of 22.3 and 35.5%, respectively [8,24]. Other reports in Sub-Saharan Africa [25] have found that AKI is mostly acquired in the community (92.1%) in our context.
Over half of our patients (58.7%) were at KDIGO stages 3 on diagnosis and similar results (57.2%) were found in Ivory Coast [26]. This could be due to the severity of the underlying disease and the late presentation of patients in our hospital. Late presentation to hospitals for AKI has been well documented in sub-Saharan Africa [25]. In this study, we identified intrinsic renal AKI (63.3%) as a major mechanism of injury with acute tubular necrosis (ATN) secondary to sepsis and nephrotoxic drugs being the most common etiology a has been previously reported elsewhere [10].
In this study, we identified the current use of traditional medicines, HIV stage 3 and 4, hypotension, and low Haemoglobin level ≤ 8.0 g/dl as potential risk factors for AKI in this subpopulation. This can be explained by the fact that in our setting, most participants (81.5%) presented at stage 3 or 4 of HIV infection, with more than two-thirds (71.8%) having anemia, which was probably due to inflammatory syndrome related to the HIV infection. The relationship between anemia and AKI has been reported in many studies [27-29]. Anemia increases the risk of AKI and worse the prognosis of AKI. The proposed mechanism could either be by an increased incidence of hypotension or a decrease in oxygen-carrying capacity [27]. In order, way AKI can favor the development of anemia by several mechanisms: severe inflammation, decreased erythropoiesis, bleeding, hemodilution, reduced red blood cell survival time, recurrent phlebotomy for blood tests, and hemolysis [28,29]. The use of Traditional medicines was also common (25.2%) and may be related to low socioeconomic status and cultural habits.
The pooled rate of dialysis requirement for AKI in the world is estimated at 2.3% [21]. In this study, 44.4% of patients with AKI in the context of HIV infection, required hemodialysis. This is much higher than the dialysis requirements for all causes of AKI in the country [24,30]. The severity of AKI in this population may explain the need [31,32]. Higher rates of dialysis need have been reported with the severity of AKI [8,25]. The pooled access rate to dialysis in Sub-Saharan Africa has improved from 17% to 47% in the period 2010-2014 [25]. Access rate in most sub-Saharan countries is dependent on the availability of a dialysis center, space and ability to pay [33]. However, despite the presence of a dialysis unit with a highly state-subsidized cost of dialysis, the access rate was very low (42.9%) compared to 72.2% and 73.2% in other regions of the country [24,30]. The early death due to the severity of the illness (43.8%) and lack of finances (37.5%) were the main reasons for non-access to dialysis.
AKI mortality mainly depends on the severity, etiology, and access to dialysis. As has been reported, we observed a significantly higher mortality rate in the AKI group compared to the non-AKI group (42.9% vs. 16.1%), and non-access to dialysis was responsible for 33.3% of deaths in the AKI participants. Our mortality rate is however higher than previous reports [4,15]. Most of the patients in our study had community-acquired AKI and late presentation may have contributed to poorer outcomes. AKI increases the length of hospital stay by 5 to 7 days with the severity of AKI and the need for dialysis being the main drivers of this increase [34-36]. We observed a longer median duration of hospital stay of 12.0 (6.0-18.0) days in participants with AKI compared to 8.0 (6.0-15.0) days in those without AKI.
At 3 months, 66.7% of survivors had achieved complete renal recovery. A similar complete recovery rate (67.2%) has been reported [4] . The pooled rate of residual chronic kidney disease has increased from 8% to 16% in adults in the period 2010 to 2014 [25].
Because this was a single-center study, its generalizability was severely limited. We can presume that these results are representative, given the prospective nature of the study and the fact that this is the region’s hub for HIV and nephrology cases. As a result of the lack of daily serum creatinine levels, we may have missed some cases of AKI that occurred during the hospitalization.
We used only the serum creatinine criteria for AKI definition probably underestimating the incidence of AKI, however, the urine output criteria for AKI definition have been shown to be less sensitive in non-ICU populations [37]. We used the absolute lymphocyte count (ALC) as a surrogate for CD4 count, which may be limiting. However a reliable relationship exists between ALC and CD4 count, and ALC has high sensitivity and high specificity in predicting CD4 count [38].
Despite the aforementioned shortcomings, our research offers several strengths. This is the only study that we are aware of that offers an updated estimate of the incidence of AKI among HIV-positive individuals in Cameroon during the test-and-treat era. This research will increase doctors’ awareness of AKI in HIV-positive patients and aid in the early detection and management of HIV-positive patients.
In conclusion, despite the improved access to combined antiretroviral therapy (c-ART) and the Test and Treat strategy in Cameroon, severe immunosuppression remains common in HIV-positive patients, which in part explains the high incidence of acute kidney injury in hospitalized HIV-infected patients. AKI is predominantly community-acquired, with over 50% of patients diagnosed in KDIGO stage 3. AKI is independently associated with the current use of traditional medicines, WHO HIV clinical stages 3 and 4, hypotension, and anemia, and it is associated with a high need for dialysis with a modest access rate. AKI is associated with higher mortality and a longer length of hospital stay. Chronic kidney disease develops in 33% of AKI survivors.
We thank all the patients who participated in this study and the staff of the different medical wards.
- Unaids. UNAIDS report on the global AIDS epidemic 2013 GLOBAL REPORT. 2013.
- Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio Monforte A, Knysz B, Dietrich M, Phillips AN, Lundgren JD; EuroSIDA study group. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003 Jul 5;362(9377):22-9. doi: 10.1016/s0140-6736(03)13802-0. PMID: 12853195.
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853-60. doi: 10.1056/NEJM199803263381301. PMID: 9516219.
- Lopes JA, Melo MJ, Viegas A, Raimundo M, Câmara I, Antunes F, Gomes da Costa A. Acute kidney injury in hospitalized HIV-infected patients: a cohort analysis. Nephrol Dial Transplant. 2011 Dec;26(12):3888-94. doi: 10.1093/ndt/gfr192. Epub 2011 May 4. PMID: 21543659.
- Fine DM, Atta MG. Kidney disease in the HIV-infected patient. AIDS Patient Care STDS. 2007 Nov;21(11):813-24. doi: 10.1089/apc.2006.0210. PMID: 18240891.
- Perazella MA. Acute renal failure in HIV-infected patients: a brief review of common causes. Am J Med Sci. 2000 Jun;319(6):385-91. doi: 10.1097/00000441-200006000-00008. PMID: 10875295.
- Li X, Zhuang S. Acute Kidney Injury in HIV Infection. J Trop Dis. 2013 Apr;1(1):101. doi: 10.4172/2329-891X.1000101. Epub 2013 Feb 25. PMID: 26798843; PMCID: PMC4718575.
- Fouda MDH, Ashuntantang G, Halle MP, Kaze F. The epidemiology of acute kidney injury in a tertiary hospital in Cameroon: A 13 months review. J Nephrol Ther. 2016; 6(3):250.
- WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children HIV/AIDS Programme. 2007.
- Franceschini N, Napravnik S, Eron JJ Jr, Szczech LA, Finn WF. Incidence and etiology of acute renal failure among ambulatory HIV-infected patients. Kidney Int. 2005 Apr;67(4):1526-31. doi: 10.1111/j.1523-1755.2005.00232.x. PMID: 15780107.
- Lopes JA, Fernandes J, Jorge S, Neves J, Antunes F, Prata MM. Acute renal failure in critically ill HIV-infected patients. Crit Care. 2007; 11(1):1–1.
- Randall DW, Brima N, Walker D, Connolly J, Laing C, Copas AJ, Edwards SG, Batson S, Miller RF. Acute kidney injury among HIV-infected patients admitted to the intensive care unit. Int J STD AIDS. 2015 Nov;26(13):915-21. doi: 10.1177/0956462414561034. Epub 2014 Nov 18. PMID: 25411349.
- Roe J, Campbell LJ, Ibrahim F, Hendry BM, Post FA. HIV care and the incidence of acute renal failure. Clin Infect Dis. 2008 Jul 15;47(2):242-9. doi: 10.1086/589296. PMID: 18540821.
- Ibrahim F, Naftalin C, Cheserem E, Roe J, Campbell LJ, Bansi L, Hendry BM, Sabin C, Post FA. Immunodeficiency and renal impairment are risk factors for HIV-associated acute renal failure. AIDS. 2010 Sep 10;24(14):2239-44. doi: 10.1097/QAD.0b013e32833c85d6. PMID: 20634665.
- Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006 Feb 28;20(4):561-5. doi: 10.1097/01.aids.0000210610.52836.07. PMID: 16470120.
- Li Y, Shlipak MG, Grunfeld C, Choi AI. Incidence and risk factors for acute kidney injury in HIV Infection. Am J Nephrol. 2012;35(4):327-34. doi: 10.1159/000337151. Epub 2012 Mar 24. PMID: 22456100; PMCID: PMC3362304.
- Silva Júnior GB, Libório AB, Mota RM, Abreu KL, Silva AE, Araújo SM, Daher EF. Acute kidney injury in AIDS: frequency, RIFLE classification and outcome. Braz J Med Biol Res. 2010 Nov;43(11):1102-8. doi: 10.1590/s0100-879x2010007500100. Epub 2010 Oct 1. PMID: 20922270.
- Choi AI, Li Y, Parikh C, Volberding PA, Shlipak MG. Long-term clinical consequences of acute kidney injury in the HIV-infected. Kidney Int. 2010 Sep;78(5):478-85. doi: 10.1038/ki.2010.171. Epub 2010 Jun 2. PMID: 20520594; PMCID: PMC3913062.
- Valeri A, Neusy AJ. Acute and chronic renal disease in hospitalized AIDS patients. Clin Nephrol. 1991 Mar;35(3):110-8. PMID: 2032395.
- Nadkarni GN, Patel AA, Yacoub R, Benjo AM, Konstantinidis I, Annapureddy N, Agarwal SK, Simoes PK, Kamat S, Menon MC, Wyatt CM. The burden of dialysis-requiring acute kidney injury among hospitalized adults with HIV infection: a nationwide inpatient sample analysis. AIDS. 2015 Jun 1;29(9):1061-6. doi: 10.1097/QAD.0000000000000653. PMID: 26125139; PMCID: PMC4489559.
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013 Sep;8(9):1482-93. doi: 10.2215/CJN.00710113. Epub 2013 Jun 6. Erratum in: Clin J Am Soc Nephrol. 2014 Jun 6;9(6):1148. PMID: 23744003; PMCID: PMC3805065.
- Izzedine H, Baumelou A, Deray G. Acute renal failure in HIV patients. Nephrol Dial Transplant. 2007 Oct;22(10):2757-62. doi: 10.1093/ndt/gfm404. Epub 2007 Jun 25. PMID: 17595186.
- Rao TK, Friedman EA. Outcome of severe acute renal failure in patients with acquired immunodeficiency syndrome. Am J Kidney Dis. 1995 Mar;25(3):390-8. doi: 10.1016/0272-6386(95)90099-3. PMID: 7872316.
- Halle MPE, Chipekam NM, Beyiha G, Fouda H, Coulibaly A, Hentchoya R, Kaze FF, Luma NH, Ashuntantang G. Incidence, characteristics and prognosis of acute kidney injury in Cameroon: a prospective study at the Douala General Hospital. Ren Fail. 2018 Nov;40(1):30-37. doi: 10.1080/0886022X.2017.1419970. PMID: 29285953; PMCID: PMC6014289.
- Olowu WA, Niang A, Osafo C, Ashuntantang G, Arogundade FA, Porter J, Naicker S, Luyckx VA. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016 Apr;4(4):e242-50. doi: 10.1016/S2214-109X(15)00322-8. PMID: 27013312.
- Yao KH, Sanogo S, Doumbia A, Konan SD, Zoue KS, Diallo AD. Acute kidney injury in hospitalized HIV-infected patients living in Cote d’Ivoire. jrenendo.com. 2018;
- Russell JA, Phang PT. The oxygen delivery/consumption controversy. Approaches to management of the critically ill. Am J Respir Crit Care Med. 1994 Feb;149(2 Pt 1):533-7. doi: 10.1164/ajrccm.149.2.8306058. PMID: 8306058.
- Han SS, Baek SH, Ahn SY, Chin HJ, Na KY, Chae DW, Kim S. Anemia Is a Risk Factor for Acute Kidney Injury and Long-Term Mortality in Critically Ill Patients. Tohoku J Exp Med. 2015 Dec;237(4):287-95. doi: 10.1620/tjem.237.287. PMID: 26607258.
- Hales M, Solez K, Kjellstrand C. The anemia of acute renal failure: association with oliguria and elevated blood urea. Ren Fail. 1994;16(1):125-31. doi: 10.3109/08860229409044854. PMID: 8184139.
- Fouda MDEH, Teuwafeu DG, Kombe F, Halle MP, Siysi VV, Abderraman G, et al. The Clinical Pattern and Outcomes of Acute Kidney Injury in a Semi-Urban Hospital Setting in Cameroon. J Nephrol Ther. 2018;8(2):1–7.
- Naicker S, Aboud O, Gharbi MB. Epidemiology of acute kidney injury in Africa. Semin Nephrol. 2008 Jul;28(4):348-353. doi: 10.1016/j.semnephrol.2008.04.003. PMID: 18620957.
- Mathew AJ, George J. Acute kidney injury in the tropics. Ann Saudi Med. 2011 Sep-Oct;31(5):451-6. doi: 10.4103/0256-4947.84620. PMID: 21911980; PMCID: PMC3183677.
- Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, Cruz D, Jaber B, Lameire NH, Lombardi R, Lewington A, Feehally J, Finkelstein F, Levin N, Pannu N, Thomas B, Aronoff-Spencer E, Remuzzi G. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015 Jun 27;385(9987):2616-43. doi: 10.1016/S0140-6736(15)60126-X. Epub 2015 Mar 13. PMID: 25777661.
- Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365-70. doi: 10.1681/ASN.2004090740. Epub 2005 Sep 21. PMID: 16177006.
- Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014 Jan;9(1):12-20. doi: 10.2215/CJN.02730313. Epub 2013 Oct 31. PMID: 24178971; PMCID: PMC3878695.
- Challiner R, Ritchie JP, Fullwood C, Loughnan P, Hutchison AJ. Incidence and consequence of acute kidney injury in unselected emergency admissions to a large acute UK hospital trust. BMC Nephrol. 2014 May 29;15:84. doi: 10.1186/1471-2369-15-84. PMID: 24885247; PMCID: PMC4046061.
- Jin K, Murugan R, Sileanu FE, Foldes E, Priyanka P, Clermont G, Kellum JA. Intensive Monitoring of Urine Output Is Associated With Increased Detection of Acute Kidney Injury and Improved Outcomes. Chest. 2017 Nov;152(5):972-979. doi: 10.1016/j.chest.2017.05.011. Epub 2017 May 17. PMID: 28527880.
- Shapiro NI, Karras DJ, Leech SH, Heilpern KL. Absolute lymphocyte count as a predictor of CD4 count. Ann Emerg Med. 1998 Sep;32(3 Pt 1):323-8. doi: 10.1016/s0196-0644(98)70008-3. PMID: 9737494.