More Information
Submitted: May 10, 2022 | Approved: May 13, 2022 | Published: May 16, 2022
How to cite this article: Alshwikh H, Alshwikh F, Elshwekh H. Persistent symptomatic hyponatremia post-COVID 19: case report. J Clini Nephrol. 2022; 6: 058-062.
DOI: 10.29328/journal.jcn.1001090
Copyright License: © 2022 Alshwikh H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hyponatremia; Kidney injury; COVID-19
Persistent symptomatic hyponatremia post-COVID 19: case report
Haifa Alshwikh1, Ferial Alshwikh2 and Halla Elshwekh3*
1Internal Medicine Specialist, Medicine Department, University of Tripoli, Tripoli, Libya
2MMBS, Medicine Department, University of Tripoli, Tripoli, Libya
3MSC, Genetic Engineering Department, Biomedical Science, Biotechnology Research Center, Tripoli, Libya
*Address for Correspondence: Halla Elshwekh, MSC, Genetic Engineering Department, Biomedical Science, Biotechnology Research Center, Tripoli, Libya, Email: [email protected]
Background: Hyponatremia associated with COVID-19 is considered an independent risk factor for a prolonged hospital stay, intensive care admission, and death, but its causes and treatment are not yet well known. Many workers attribute hyponatremia associated with COVID-19 to acute kidney injury and nephropathy associated with the disease. Others suggest that it is related to the syndrome of inappropriate antidiuretic hormone secretion, sepsis, or hypothalamic-pituitary dysfunction. We report a case of persistent acute hyponatremia in a COVID-19 patient with multifactorial etiology.
Case presentation: A managed 77 years with known hypertension, type II DM, ischemic heart disease, chronic kidney disease (stage 3B and on treatment) presented with post-COVID-19 pneumonia, confusion, fever, generalized fatigability, dizziness, and lower limb edema. COVID-19 ad has been diagnosed two weeks earlier with a positive nasopharyngeal swab and was managed with dexamethasone, 10 mg oral for 10 days, azithromycin, 500 mg once orally, and levofloxacin, 500 mg once orally. At presentation, laboratory investigation showed hyponatremia (127.7 mg/dl).
Conclusion: The etiology of hyponatremia associated with COVID-19 is different from that in other cases of hyponatremia and its management should be individualized according to patient history and clinical assessment, and effort is needed to determine the exact cause.
Recently, the World Health Organization (WHO) announced COVID-19 as a pandemic, but the factors related to its evolution are not yet elucidated, and its clinical picture and complications are variable but still not well understood [1]. The infection results in bilateral, diffuse pneumonia, which causes acute respiratory disease, but it can also affect other organs, such as the kidneys, brain, and the heart [2].
Hyponatremia associated with COVID-19 has been reported recently. In a study conducted in the New York health system on 10,000 patients in 13 hospitals, disturbance of sodium level was observed in 51.7% of the patients, most of whom were hyponatremic. Hyponatremia was shown to be an independent risk factor for a prolonged hospital stay, intensive care admission, and death [3-5] but its causes and treatment are not yet known. Many hypotheses have related it to acute kidney injury and the nephropathy associated with COVID-19. Others suggested the syndrome of inappropriate antidiuretic hormone secretion (SIADH) as the most probable etiology; sepsis and hypothalamic-pituitary dysfunction have also been suggested [6]. We report a case of persistent hyponatremia post-COVID-19 with multifactorial etiology.
A 77-years-old man presented as a case of post-COVID-19 secondary pneumonia with cough, fever, generalized fatigability, dizziness, and lower limb edema. He had hypertension and has been treated with valsartan and thiazide, as well as type II diabetes mellitus being treated with diamecrone and acarbose tablets. He was also known to have ischemic heart disease post stenting and was on aspirin, atorvastatin, and concor. Moreover, he was receiving feboxstate for gout arthritis, had benign prostatic hypertrophy, osteoarthritis post bilateral total knee replacement, and was being treated for stage 3B chronic kidney disease.
His COVID-19 infection was diagnosed by a positive nasopharyngeal swab two weeks before the presentation and was being managed with dexamethasone 10 mg oral for 10 days, and azithromycin and levofloxacin courses. His vital signs on admission were as follows: blood pressure 110/70 mmHg, O2 99%, temperature 38 °C, and pulse rate 55 bpm. Physical examination showed normal jugular venous pressure, slightly decreased air entry mainly on the right side, and lower limb edema up to mid-shin.
Diagnostic assessments: The patient’s laboratory results on admission included significant neutrophilia 13 (92%) with lymphopenia (reaching 6%), iron deficiency anemia (10.2 g/dl), mild hyponatremia (134.4 mg/dl), high CRP (17.7 mg/l), high renal function test, urea 56 mg/dl, creatinine 1.9 mg/dl, D-dimer 1.19 mg/l, LDH 312 U/l, RBS 278 mg/dl, and normal procalcitonin 0.3 ng/ml (normal range < 0.5 ng/ml) (Table 1).
| Table 1: Laboratory data of the patient. | ||||||||||
| leucocyte x106/µl | 13.2 | 9.1 | 15.6 | 10.5 | 6.8 | 5.8 | 5.5 | 8.4 | 7.8 | 5.2 |
| Neutrophils% | 92% | 91% | 85% | 86% | 81% | 77% | 75% | 73% | 66% | 59% |
| Lymphocyte % | 7% | 6% | 10% | 11% | 10% | 12% | 19% | 20% | 26% | 33% |
| Hemoglobin (g/dl) | 11.2 | 10.7 | 10.2 | 9.4 | 9.7 | 8.5 | 8.9 | 8.7 | 9.4 | 9.86 |
| Platelets (109/L) | 254 | 231 | 249 | 152 | 229 | 363 | 359 | 404 | 191 | 212 |
| Na+ (mg/dl) | 134 | 128.9 | 127.5 | 127.3 | 126.2 | 123 | 124.3 | 124 | 130 | 137 |
| K+ (mg/dl) | 3.9 | 3.8 | 3.7 | 3.49 | 3.8 | 3.4 | 3.3 | 3 | 3.8 | 4.4 |
| Cl̶̶ (mg/dl) | 99.9 | 91.2 | 95.9 | 96.2 | 95.2 | 102 | 85 | 91 | 100 | 103 |
| Calcium(mg/dl) | 8.3 | 9.2 | ||||||||
| Phosphorus(mg/dl) | 4.67 | 3.6 | ||||||||
| Magnesium(mg/dl) | 1.2 | |||||||||
| Blood sugar profile | 278 | 200 | 114 | 110 | 146 | 129 | 150 | 148 | 174 | 111 |
| Creatinine (mg/dl) | 1.9 | 2.2 | 2 | 2.1 | 1.8 | 1.9 | 2 | 1.6 | 1.3 | 1.3 |
| Urea (mg/dl) | 56 | 88 | 104 | 113 | 125 | 90 | 81 | 57 | 76 | 72 |
| CRP (mg/l) | 17.7 | 5.9 | 0.32 | 90 | 82.7 | 64.7 | 48.2 | 37.3 | 10.4 | 1 |
| PT | 30.9 | 18.4 | 17.5 | 17.9 | 15.3 | 21.9 | ||||
| INR | 3.96 | 1.7 | 1.6 | 1.66 | 1.3 | 2.2q1 | ||||
| D DIMER(mg/l) | 1.19 | 0.5 | 0.54 | 0.6 | 0.64 | 0.63 | 0. 78 | 1.76 | 1.98 | 1.94 |
| Ferritin (ng/ml) | 104.1 | 139.9 | 118 | 107.2 | 107.4 | |||||
| Troponin I (ng/ml) | < 0.100 | 93.5 | ||||||||
| CK MB (U/l) | 10.5 | |||||||||
| LDH (U/L) | 279 | 312 | 415 | 396 | 346 | |||||
| HbA1C | 7.70% | |||||||||
| 25HYDROXY VIT D (ng/l) | 23.5 | |||||||||
| VITAMIN B 12(pg/ml) | 510.8 | |||||||||
| Procalcitonin (ng/ml) | 0.3 | |||||||||
Therapeutics interventions: The patient was admitted and treated with intravenous ceftriaxone, Lasix, and insulin, along with enoxaparin 0.4 subcutaneous injection twice daily, and all nephrotoxic drugs were withheld. After five days on ceftriaxone, his CRP started to increase, so the antibiotic was switched to ceftazidime 1 gram 8 hourly for 10 days. The patient started to improve, his fever subsided, CRP dropped to 0.3 mg/l, and he was discharged.
Follow-up and outcomes: After four days, the patient was brought by his family with confusion during the previous two hours, nausea and poor appetite, as well as hematuria for two days. His neurological examination was normal except for recurring delirium. He had no fever, chills, or cough, his vital signs along with general examination were normal except for massive lower limb edema. His RBS was 140 mg/dl and oxygen saturation was 96%. He denied any history of low fluid intake, use of new medications, or change in his urine habit other than the red urine.
Laboratory tests on the second presentation showed hyponatremia (127.7 mg/dl), neutrophilia 15.6 (85%), lymphopenia (7%), high CRP (90 mg/l), high LDH (415), urea (113 mg/dl), creatinine (2.2 mg/dl), HbA1c (7.2%), chloride (85.7 mmol/l), potassium (3.3 mg/dl), calcium (8.3 mg/dl), phosphorus (4.6 mg/dl), and low magnesium (1.2 mg/dl) (Table 1).
Duplex ultrasound of both legs showed no evidence of deep vein thrombosis, echocardiography showed reserved LV function (ejection fraction 75%), reversed E/A ratio, and mild mitral regurgitation. The right ventricle was normal and no thrombus or effusion was observed. A computerized scan of the brain showed no evidence of cerebral hemorrhage or infarction. MRI of the brain showed mild brain atrophy but no ischemia or hemorrhage (Figure 1).
Figure 1: Normal T1 and T2 magnetic resonance images of the brain.
Further workup for hyponatremia revealed urine osmolarity of 296.5 mOsm/kg, urinary sodium of 64.1 mEq/L, and low serum osmolality (240 mOsm/kg). Thyroid-stimulating hormone and adrenocorticotropic hormone stimulation tests were normal.
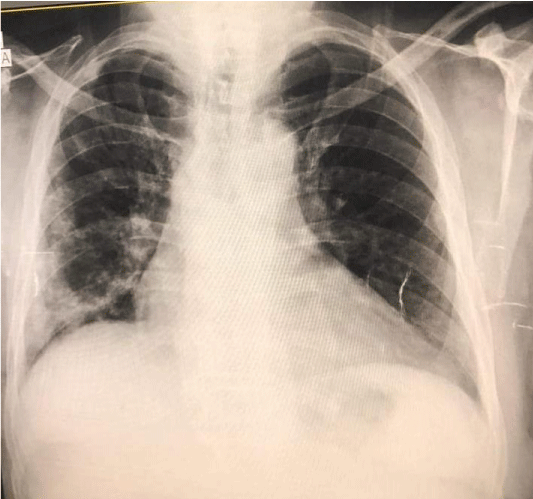
The patient was admitted to intensive care and a slow infusion of hypertonic saline (3%) was initiated, but his serum sodium level continued dropping, reaching 124 mEq/L. The next day, fluid intake was restricted to 800 ml per day for possible SIADH, and input and output were monitored daily. Serum sodium fluctuated between 124 and 127 mg/dl for the next few days, so evaluation for sepsis as a possible cause of his hyponatremia was started. The urine study showed hematuria (erythrocytes, 35/HPF) and albuminuria (ACR 700 mg in 24 h), but no pus cells or bacterial growth was observed in the urine culture. A blood culture was not done because the patient had received many antibiotics. Intravenous meropenem was started with human albumin and furosemide, along with insulin and 0.4 units of enoxaparin twice daily. A chest X-ray followed with high-resolution computerized tomography of the chest revealed radiological features of atypical viral pneumonia, bilaterally but mainly on the right side (post COVID-19 pneumonia residual changes), but no new infiltrates or consolidation Figures 2,3, CRP started dropping and the patient was improving. Subsequent laboratory investigation showed gradual normalization of serum sodium to 137 mg/dl over two weeks, blood urea to 55 mg/dl, creatinine to 1.4 mg/dl, and CRP to 1 mg/l.
Figure 2: Chest X-ray posterior lateral view of a patient with COVID-19 pneumonia, both lungs are showing signs, particularly the right (The New Bridge Hospital, Tripoli, Libya).
Figure 3: Axial high resolution CT image shows multiple focal peripheral opacities in the lateral segment of the right middle lobe (The New Bridge Hospital, Tripoli, Libya).
Our case study adds more evidence of the associated electrolyte disturbances with COVID-19 infection. The patient exhibited hyponatremia, hypokalemia, and hypomagnesemia, suggesting that he was admitted with acute kidney injury on top of chronic kidney disease. A Chinese study on 12 patients also found an association between COVID-19 and electrolyte imbalance (half of the patients had hypokalemia and the other half hyponatremia) and that was correlated with renal injury [7,8]. Another observation of 5449 patients with COVID-19 in New York reported that 36.6% of them presented with acute kidney injury [9,10]. Thus, attributing our patient’s hyponatremia to SIADH would be controversial.
Many previous analyses linked hyponatremia in COVID-19 patients to SIADH in the presence of pneumonia due to the secretion of pro-inflammatory cytokines such as IL-1b and IL-6, which can stimulate hypothalamic arginine vasopressin release. In a study on 594 COVID-19 patients with hyponatremia, it was attributed to SIADH in 83.8% of the patients [7]. Based on the available data on our patient, identifying the cause of the hyponatremia is difficult. On the one hand, laboratory data (serum osmolality < 275 mOsm/ kg, urine osmolality > 100 mOsm/kg, urine sodium concentration > 40 mEq/l) point to SIADH. Moreover, pneumonia and previous treatment with medications known to be implicated in SIADH secretion, such as azathioprine and levofloxacin, support that hypothesis. On the other hand, this hypothesis is contradicted by the lower limb edema on admission and the previous history of chronic kidney disease, as well as the failure of fluid restriction measures in overcoming the hyponatremia.
In looking for other possible explanations of hyponatremia in COVID-19 patients, we found a case report on a Filipino man 52 years of age with COVID-19 and admitted with hypovolemic hyponatremia (NA: 108 mg/dl) due to gastrointestinal tract loss. The workup for hyponatremia excluded SIADH as a cause and the patient required fluid replacement to normalize his sodium level [11].
Our case report supports other analyses suggesting that chronic kidney disease, advanced age, bilateral lung involvement, and previous use of antibiotics implicated in the development of SIADH, such as azithromycin and levofloxacin, are all associated with high-frequency rates of hyponatremia among COVID-19 patients [12,13].
This patient was admitted with high temperature and elevated CRP which put the sepsis as a probable cause of his hyponatremia but we lack other supporting laboratory pieces of evidence, fever and high CRP in a hyponatremic patient with COVID-19 was documented previously in other studies which found a significant association between the two variables (p = 0.040) [5].
Another explanation for our case hyponatremia is COVID-19 nephropathy given that the patient was presented with hematuria and macroalbuminurea of 700 mg over 24 hours, but a kidney biopsy was not taken due to lack of resources, so a definitive diagnosis could not be made. Similar presentations have been documented in a series of six patients with COVID-19 who developed proteinuria. Their renal biopsy showed podocytopathy, and collapsing glomerulopathy [14], others found massive albuminuria in 34% of 59% COVID-19 patients on day one of admission [15].
We could not attribute the hyponatremia in our patients to a single cause. Rather, we cite different possible mechanisms, including SIADH, sepsis, acute kidney injury, probable COVID-19 associated nephropathy, and use of thiazide, azathioprine, and levofloxacin. The only way we could normalize this patient’s serum sodium was by focusing on managing the secondary post COVID-19 infection per se with broad-spectrum antibiotics and maintaining volume status.
More analyses are needed to investigate electrolyte imbalances associated with COVID-19, especially hyponatremia, which is being observed more frequently. More knowledge is needed of its causes and its relation to disease severity, morbidity, and mortality, and guidelines for its management should be developed.
Study limitations
The main limitation in this case report is that though the patient presented with lower limb edema, his volume status was not assessed properly. Furthermore, sepsis was diagnosed clinically: blood culture was not done, lactate level was not measured, and kidney biopsy was not obtained though it is considered mandatory in such cases.
Hyponatremia associated with COVID-19 has different etiologies and its management should be individualized according to the patient’s history and clinical assessment. The decision of fluid restriction or replacement should take into consideration managing the COVID-19 secondary infection itself.
Declaration
Ethics approval and consent to participate: Ethical approval was granted by BTRC (Ref No: BEC-BTRC 27-2021), Patient consent was obtained from the patient.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Authors’ contributions: All authors contributed to the conception and design of the study, data acquisition and analysis, and writing and revising of the manuscript. Both of them approve the publication of the manuscript.
Acknowledgment: Many thanks to the BTRC head Prof. Adam Elzagheid for supporting us in publishing this case report.
- José Carlos de la Flor M, Francisco Valga A, Alexander M, Miguel Rodeles del P. Hyponatremia in COVID-19 Infection - Possible Causal Factors and Management. J Clin Nephrol Ren Care. 2020;6(2):53–6.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-1069. doi: 10.1001/jama.2020.1585. Erratum in: JAMA. 2021 Mar 16;325(11):1113. PMID: 32031570; PMCID: PMC7042881.
- Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Malieckal DA, Bellucci A, Sachdeva M, Rondon-Berrios H, Jhaveri KD, Fishbane S, Ng JH; Northwell Nephrology COVID-19 Research Consortium. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. 2021 May 27;36(6):1135-1138. doi: 10.1093/ndt/gfab067. PMID: 33724428; PMCID: PMC7989196.
- Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, Dewsnip A, Falayi K, McCaughran W, Mullins C, Naeem A, Nwokolo M, Quah H, Bitat S, Deyab E, Ponnampalam S, Bouloux PM, Montgomery H, Baldeweg SE. Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19. J Clin Endocrinol Metab. 2021 May 13;106(6):1637-1648. doi: 10.1210/clinem/dgab107. PMID: 33624101; PMCID: PMC7928894.
- De Carvalho H, Letellier T, Karakachoff M, Desvaux G, Caillon H, Papuchon E, et al. Hyponatremia is associated with poor outcome in COVID-19. J Nephrol [Internet]. 2021;(0123456789). Available from: https://doi.org/10.1007/s40620-021-01036-8
- Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong AS. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005 Aug 1;202(3):415-24. doi: 10.1084/jem.20050828. Epub 2005 Jul 25. PMID: 16043521; PMCID: PMC2213088.
- De Carvalho H, Richard MC, Chouihed T, Goffinet N, Le Bastard Q, Freund Y, et al. Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: a case–control study. Intern Emerg Med [Internet]. 2021;(0123456789). https://doi.org/10.1007/s11739-021-02632-z
- Hong X, Chi Z, Liu G, Huang H, Guo S. Analysis of early renal injury in COVID-19 and diagnostic value of multi-index combined detection. 2020;1–23.
- Meijers B, Hilbrands LB. The clinical characteristics of coronavirus-associated nephropathy. Nephrol Dial Transplant. 2020 Aug 1;35(8):1279-1281. doi: 10.1093/ndt/gfaa197. PMID: 32871591; PMCID: PMC7499749.
- Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 Jul;98(1):209-218. doi: 10.1016/j.kint.2020.05.006. Epub 2020 May 16. PMID: 32416116; PMCID: PMC7229463.
- Khan AA, Ata F, Munir W, Yousaf Z. Fluid Replacement Versus Fluid Restriction in COVID-19 Associated Hyponatremia. Cureus. 2020 Jul 8;12(7):e9059. doi: 10.7759/cureus.9059. PMID: 32782878; PMCID: PMC7413320.
- Hu W, lv X, Li C, Xu Y, Qi Y, Zhang Z, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med [Internet]. 2020;(0123456789). https://doi.org/10.1007/s11739-020-02515-9
- Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, Romero R, Becerra-Muñoz VM, Uribarri A, Feltes G, Trabattoni D, Molina M, García Aguado M, Pepe M, Cerrato E, Alfonso E, Castro Mejía AF, Roubin SR, Buzón L, Bondia E, Marin F, López Pais J, Abumayyaleh M, D'Ascenzo F, Rondano E, Huang J, Fernandez-Perez C, Macaya C, de Miguel Novoa P, Calle-Pascual AL, Estrada Perez V, Runkle I; HOPE COVID-19 investigators. Prognostic Impact of Hyponatremia and Hypernatremia in COVID-19 Pneumonia. A HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry Analysis. Front Endocrinol (Lausanne). 2020 Nov 30;11:599255. doi: 10.3389/fendo.2020.599255. PMID: 33329400; PMCID: PMC7734292.
- Shetty AA, Tawhari I, Safar-Boueri L, Seif N, Alahmadi A, Gargiulo R, et al. COVID-19–Associated Glomerular Disease. J Am Soc Nephrol [Internet]. 2021 Jan 1;32(1):33 LP – 40. http://jasn.asnjournals.org/content/32/1/33.abstract
- Widiasta A, Sribudiani Y, Nugrahapraja H, Hilmanto D, Sekarwana N, Rachmadi D. Potential role of ACE2-related microRNAs in COVID-19-associated nephropathy. Non-coding RNA Res [Internet]. 2020;5(4):153–66. https://www.sciencedirect.com/science/article/pii/S2468054020300561