More Information
Submitted: March 11, 2022 | Approved: May 09, 2022 | Published: May 10, 2022
How to cite this article: Kaur KK, Allahbadia G, Singh M. An update on the approaches of avoidance of propagation of chronic kidney disease resulting in reversal or possible need or avoidance of kidney transplantation - a systematic review. J Clini Nephrol. 2022; 6: 040-057.
DOI: 10.29328/journal.jcn.1001089
Copyright License: © 2022 Kaur KK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
An update on the approaches of avoidance of propagation of chronic kidney disease resulting in reversal or possible need or avoidance of kidney transplantation - a systematic review
Kulvinder Kochar Kaur1* , Gautam Allahbadia2 and Mandeep Singh3
, Gautam Allahbadia2 and Mandeep Singh3
1Scientific Director, Dr. Kulvinder Kaur Centre for Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
2Scientific Director, Ex-Rotunda - A Centre for Human Reproduction, 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra (W)-400040, Mumbai, India
3Consultant Neurologist, Swami Satyanand Hospital, Near Nawi Kachehri, Baradri, Ladowali road, Jalandhar, Punjab, India
*Address for Correspondence: Dr. Kulvinder Kochar Kaur, M.D., Scientific Director, Dr. Kulvinder Kaur Centre For Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India, Email: [email protected]
Chronic Kidney Disease (CKD) by definition is a disease characterized by irreversible elimination of renal function, which keeps propagating as corroborated by an estimated glomerular filtration rate (eGFR) of < 60 ml/min/1.73m2, the constant existence of presentation which pointed to Kidney injury (proteinuria, active sediments of urine, histological injury, structural aberrations or prior history with regards to Kidney transplantation) or both that are persistent for greaterthan 3 mths [1]. CKD has continued to be a worldwide Public Health problem, as well as comprises considerable healthcare along with cost burden, since a reduction in GFR has been understood to result in, escalation of Diabetes mellitus (DM), hypertension cardiovascular processes, hospital admissions, cognitive impairment in addition to total mortality [2]. The prevalence of CKD has a variability as per the geographic region that varies from 10% - 20%, however, escalates slowly in particular in developed countries [3]. Partly this pattern might be secondary to the enhancement of the aging population all over the globe [4]. Additionally, the escalation of the prevalence of risk factors like DM, hypertension along with obesity in patients with CKD is noticeable [5].
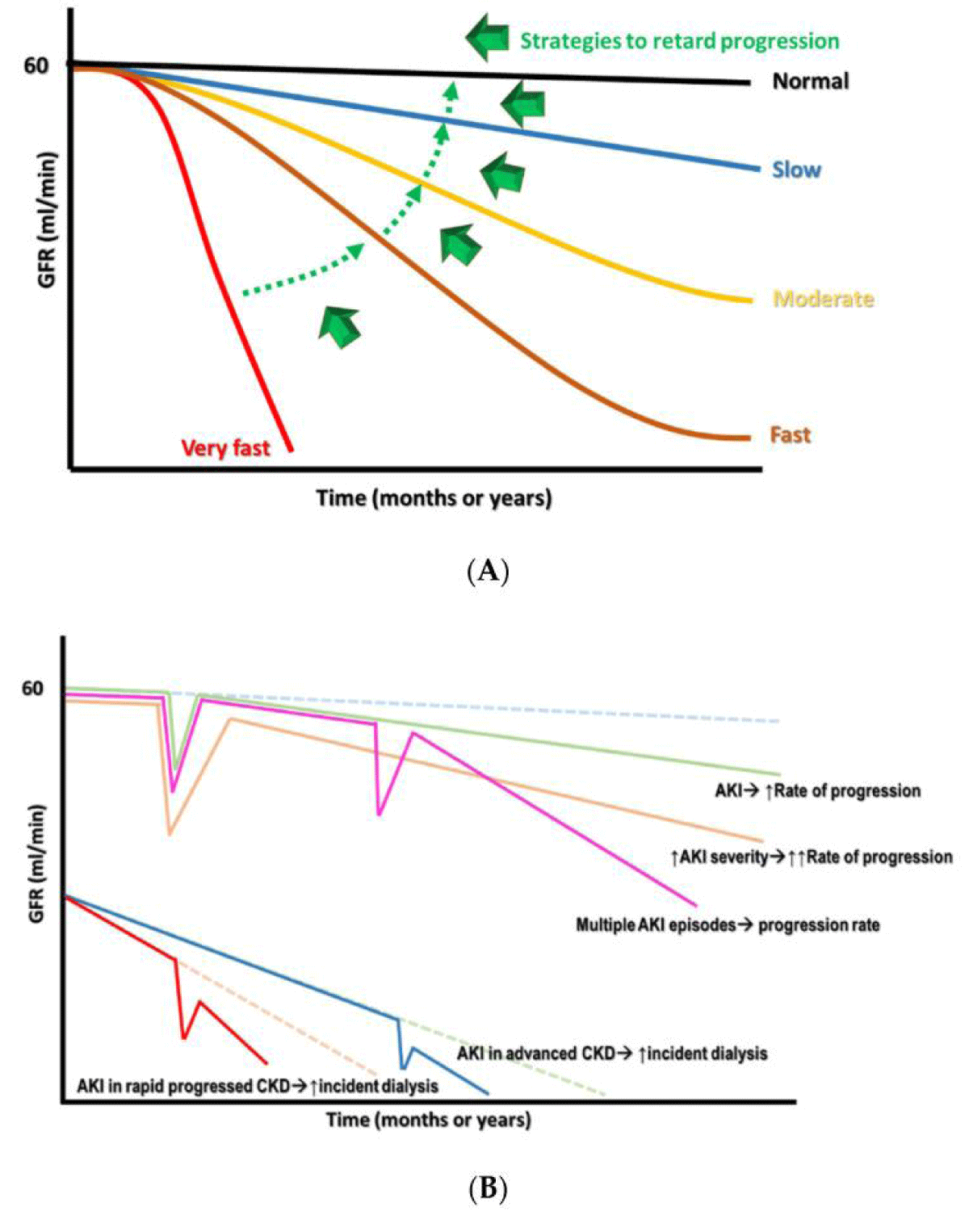
Once a diagnosis of CKD is made it implies that the person’s renal function has reached a time of becoming irreparable which suggests that the reduction of renal function within a time period is not avoidable along with commonly irremediable. Nevertheless, still variable patterns of renal function reduction in patients with CKD are existent. The classification of these patterns can be made automatically into i) very fast, ii) fast, iii) moderate or iv) slow based on the threshold needed for definition, the rate of reduction of renal function with the utilization of ml/min/year or ml/min/month (Figure 1A) [6]. These variations in patterns are mainly correlated with the heterogeneity of CKD initiation, the following pathologies, associated comorbidities, and therapies patients get in addition to exposure to difficult microenvironments [7]. After reviewing epigenetic modifications in, DKD, Vit C in AKI avoidance, the role of VitD in renal diseases here our concentration is on possible approaches which have been generated with the objective of reduction of the aggravation of renal function as well as treatment of the particular significant complications in patients who present with CKD and thus the requirement for renal transplantation.
Figure 1: Courtesy ref no-6-(A) The variability of reduction rates of renal function in CKD with the target shift from very rapid to slow rate. (B) The sequelae of AKI on CKD propagation, based on the robustness as well as the commonality of the events.
Here we conducted a systematic review utilizing the search engine PubMed, Google scholar; web of science; Embase; Cochrane reviews library utilizing the MeSH terms like CKD propagation; risk factors; eGFR; Diabetic Kidney Disease (DKD; other etiologies of CKD; epithelial-mesenchymal transition (EMT); FSGS; proteinuria; Avoidance of Acute Kidney Injury (AKI); etiology for AKI; glomerulosclerosis; glomerulopathy; hypertension with CKD; Heart failure (HF) with CKD; Nephrolithiasis & urolithiasis; renal anemia; metabolic acidosis; Hyperkalaemia from 2000 till date in 2022. See Figure 1 for selection criteria.
We found a total of 183,000 articles on CKI of which 250 were correlated with CKD propagation out of which we selected 139 articles for this review. No meta-analysis was done.
CKD propagation risks
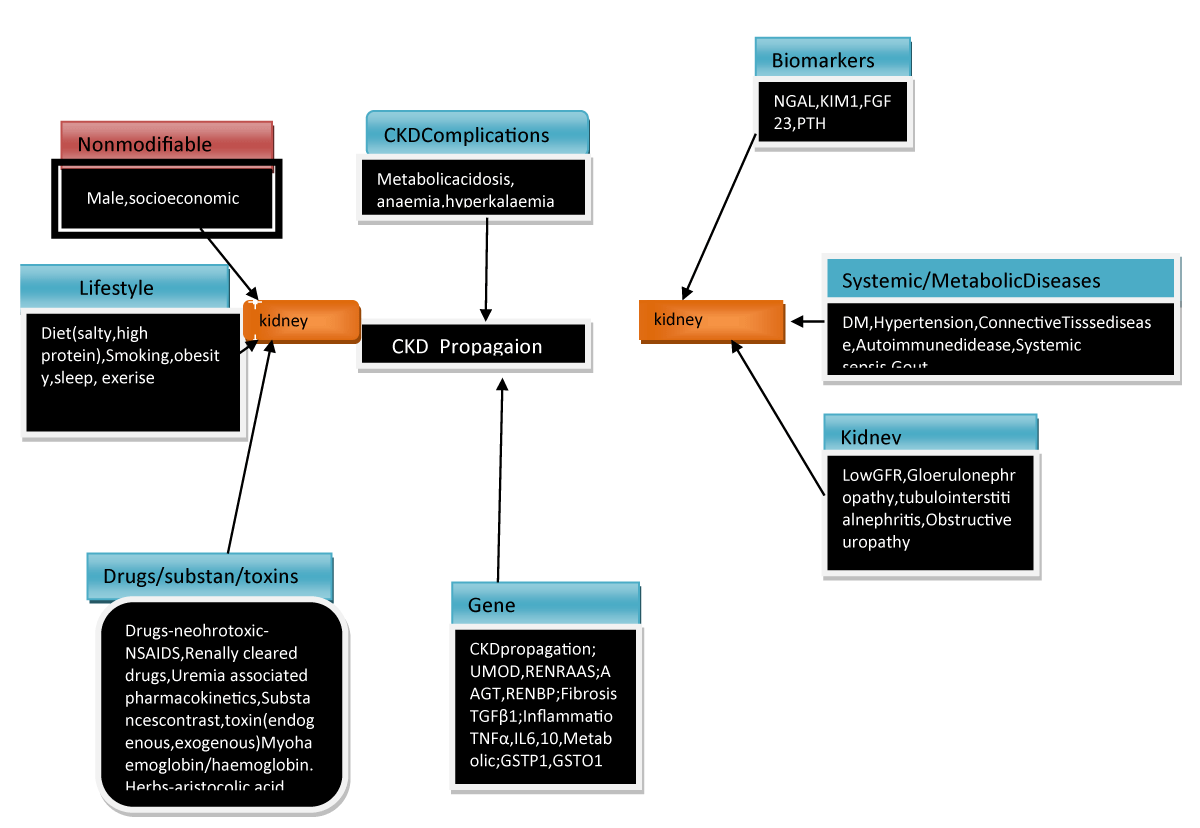
A remarkable difference in CKD was observed in the determined GFR track that implied the existence of considerable heterogenous risk factors that might aid in the propagation of CKD besides these risk factors might be the probabilistic treatment targets for conferring renoprotection. We already possess the knowledge that the significant risk factors that usually account for CKD propagation are socioeconomic parameters inclusive of lifestyles factors like diet, sleep deprivation, smoking, and absence of exercise which result in CKD propagation. Systemic in addition to metabolic situations inclusive of DM, hypertension, and cardiovascular disease (CVD) might further result in precipitation along with exacerbating eGFR deterioration (Figure 2) [8]. Furthermore recently atrial fibrillation (AF) has been demonstrated to aid in accelerated eGFR deterioration as well as the CHA2D S2-VAScscore, a stroke risk classification model for the patients with AF can result in anticipation of renal propagation [9].
Figure 2: Courtesy ref no-6-The risk factors and management approaches of CKD generation and propagation.
Moreover the intrinsic factors further possessed a significant role in correlation with Kidney in impacting the deterioration of renal function like GFR, proteinuria, glomerulopathy, interstititial damage along with renal outlet blockade (alias obstructive nephropathy). Every glomerulopathy uniquely impacts the rate of reduction of renal function like the focal segmental glomerulosclerosis (FSGS) possess greater chance of accelerated eGFR reduction, whereas the rate is lesser for IgA nephropathy (IgAN), membraneous nephropathy (MN) as well as Diabetic Kidney Disease (DKD) [10]. Gene polymorphisms in those implicated in pathways like the ones correlated with inflammatory responses; (like Tumor necrosis factor alpha (TNFα), interleukin-4 (IL4), fibrosis; like trans forming growth factor beta (TGF-β), phase II metabolism; (like GSTP1 as well as GSTO1) along with, Renin-Angiotensin –aldosterone System (RAAS) (like AGT as well as RENBP) have been pointed to influence the propagation rates of CKD [11].
Epithelial –Mesenchymal Transition (EMT)
Renal fibrosis inclusive of nephrosclerosis as well as tubulointerstitial fibrosis comprises the ultimate pathway that is shared for renal damages irrespective of the causation. EMT represents the main mode that results in facilitation of renal fibrosis along with myofibroblasts are the major cell kinds which cause generation of extracellular matrix (ECM) [12]. The initiation of myofibroblasts within the Kidney is not clear however, different candidates have been pointed that is inclusive of Bone marrow obtained fibroblasts, or during transit from pericytes to endothelial cells [13]. More recently work has documented that EMT is not that common since fibroblasts obtained from EMT are only occasionally observed in the renal interstitium. An innovative belief of partial EMT, that pointed that tubular epithelial cell acquire mesenchymal properties, however keep their attachment with the basement membrane (BM), might reason out the pathogenic part of renal tubular epithelial cells in case of renal fibrosis [14].
Subsequent to acute Kidney injury (AKI) insults the c-jun NH2termial kinase (JNK) signal activation in tubular epithelial cells for escalation of the expression of classical mesenchymal markers (like e-cadherin, α-smooth muscle actin) in addition to up regulation of profibrotic factors (basically TGF-β1) along with Connective tissue growth factor (CTGF) [15]. The continuous activation of the TGF-β1 pathway that results in an escalation of SNAI1 or TWIST1 leads to further facilitation of G2/M arrest. Arrest of the cell cycle in the G2/M phase in case of damaged tubular epithelial cells, via activation of JNK results in exaggeration of the profibrotic factors like TGF-β1 as well as Connective tissue growth factor (CTGF) that ultimately ends in a vicious cycle that causes propagation of fibrosis [16]. Fatty acids oxidation (FAO) serves as the major energy provider to the proximalconvoluted tubule (PCT). The activation of SMAD 3 by TGF-β1 would cause repression of expression, of PPARGC1α that results in impairment of FAO with resultant lipid accrual in the PCT that is a property of EMT [17]. This lipid accrual in the PCT would cause escalation of inflammation, innate immunity along with apoptosis to deteriorate the renal fibrosis further. Tubular cells that possess partial EMT further possess the capacity of activation of fibroblasts along with cause recruitment of inflammatory cells through the secretome constituted of growth factors, cytokines as well as chemokines which is followed by worsening of fibrosis [18]. Strategies that target the cell cycle or result in hampering of SNAI1 or TWIST1 expression to block the EMT might work as a good target for the reverting of the renal fibrosis [13,16]. Bone morphogenetic protein 7 (BMP 7) possesses the capacity of reverting EMT by counter action of the TGF-β1/SMAD2/3 pathway along with acts as one more probability of targeting for treatment or enhancement of renal damage [19]. Nevertheless, the outcomes of the clinical studies of BMP 7 analogs implicating patients with CKD are heterogenous that pointed to a complicated crosstalk amongst BMP 7 along with other EMTassociated pathways in addition to the need of estimation of the ideal serum BMP 7 amounts [20].
Epigenetic modifications that are inclusive of DNA methylation along with histone post-translational modifications further have a robust role in controlling partial EMT. The hampering of DNA methylation was documented to mitigate renal fibrosis. Like low dose hydralazine that resulted in demethylation of the NASAL1 promoter along with 5azacytidine caused the hampering of DNA methyltransferase (DNMT) [21]. Moreover substances that targeted histone modifications conferred further renal advantages in CKD or AKI-CKD transformation via the hampering of histone methyltransferase (like enhancer ofzene homolog2 (EZH2) or the hampering of histone deacetylases (like valproic acid)or indirectly interfere with the histone modification readers (bromodomain as well as extra terminal (BET) protein Inhibitors [22-24]. Thus epigenetics might be an innovative treatment target for renal diseases.
Avoidance of Acute Kidney Injury (AKI) in CKD Patients
Acute Kidney injury (AKI) is correlated with considerable morbidity along with mortality that is inclusive of an escalation of side effects associated with renal results. The accrued proof pointed that AKI is never restricted by itself, since it works in the form of a gateway following AKI events along with probability of incident CKD irrespective of if patients demonstrated improvement from the AKI incident or not [25]. Furthermore, a lone episode of event that was superimposed on patients that possessed preexistent CKD could result in greater renal aggravation towards end stage renal Disease (ESRD) fast–at a nonlinear speed (Figure 1B). The risk factor correlated with the AKI associated enhancement of renal propagation had been an observation earlier in addition to were inclusive of elderly patients, postponement in renal rehabilitation from AKI, robust AKI experience, the existence of proteinuria along with comorbidities like DM, hypertension, heart failure (HF) [26]. Longterm renal complication can become quiet critical if patients possess acute tubular necrosis (ATN) or ischemic AKI in contrast to those have other types of AKI, pointed that the causative factor of AKI might further have a remarkable significance as a risk factor [27].
Biomarkers like Kidney injury molecule (KIM) 1 along with neutrophils gelatinase associated lipocalin (NGAL) further possess the capacity of estimaton of AKI prior to the standard pointers [28]. Furthermore, a combination of biomarkers like insulin like growth factor binding protein7 ( IGFBP7), as well as Tissue inhibitors of Matrix Metalloproteinases2 (TIMP2)were illustrated to cause anticipation of the generation of AKI at 12h subsequent to blood tests along with dictate the selection of the relevant treatment for avoidance of AKI [29].
The mode implicated in transit from AKI to CKD is still going through assessment with a response that is maladaptive along with partial EMT in particular at the –PCT possesses a significant part. Cell cycle arrest in the G2/M phase in which impairment of regeneration of the damaged PCT secondary to mitochondrial impairment as well as abnormalitiesof activation of the generational pathways (like Wnt, Hedgehog along with Notch pathways)further aid in the AKI to CKD transit. Phenotypic alterations of the fibroblasts to myofibroblasts or the production of tertiary lymphoid tissues, in addition to dysregulated switches of the T cells having undergone recruitment along with M1 macrophages to regulatory T cells along with M2 macrophages respectively would prolong inflammation along with fibrosis that results in reduction of density of the capillaries, hypoxia in addition to tubular cell injury that comprises of a vicious cycle [30]. Moreover the continued expression of TGF-β1 or KIM1 molecule has further been implicated [31]. Noticeably variable pathogenic events have role in aiding AKI to CKD transit are further physiological healing events amongst the kidneys. Further insults in the form of salt or high protein consumption along with nephrotoxic substances at the time of or subsequent to AKI events in addition to the underlying pathological event (like Diabetic nephropathy) might alter the normal physiological renal reaction to impairment in regeneration.
B isolation of etiological factors for AKI
The foundational management of AKI in patients with CKD implicates avoidance of the relapse of AKI in addition to the recognition of the etiologies aiding in this AKI. Moreover treatment of these etiological parameters implicated besides the maximization of volume along with haemodynamic removal of the nephrotoxic substances, the manipulation of the dosages of the medicine as per the renal function with utilization of a conservative strategy (< 180 mg/dl) in contrast to augmentation of glycaemic regulation in addition to sustenance of greater arterial pressure in patients undergoing therapy for hypertension can prove to be the key approaches towards management of AKI [32]. Drug treatments are required to get rechecked for avoidance of any drug-drug crosstalk. Like addition of piperacillin/tazobactum has to be prevented in view of it causing the escalation of nephrotoxicity of vancomycin. Different substances along with approaches have been evaluated for the treatment inclusive of recombinant alkaline phosphatase along with L carnitine for the occurrence of sepsis associated AKI, besides p53 targeted small interfering RNA (siRNA) along with distant ischemic prior conditioning with regards to surgery correlated AKI [33].
An approach of treatment dependent on etiology for CKD
The variation in causes of CKD by themselves further influence the renal propagation of CKD. Here the etiology dependent treatment is just briefly summarized with the topic not having been well tackled for both the usual along with lesser known causes of CKD in the context of CKD.
Glomerulopathy: Glomerulopathy represents a heterogenous group of diseases that is responsible for a considerable amounts of CKD. It takes place usually in younger patients population with the manifestation being not particular. Despite innovative approaches being under assessment renal biopsy continues to be the gold standard for attaining a confirmed diagnosis. A slow decline in renal function takes place in a percentage of patients. The risk factors for a greater rapid GFR fall are inclusive of obesity, smoking, hypertension, remarkable proteinuria (mostly > 1 g daily). CKD, once the diagnosis has been established in the context of glomerulopathy along with pathological chronic renal abrasions (glomerulosclerosis, tubular atrophy as well as interstitial fibrosis [34]. Genetic factors aid in a fast GFR reduction like APOL1 in the ones with FSGS [35]. Fabry Disease gets commonly not appreciated an X-linked heritable disease whose etiology is a pathogenic mutation that implicates GLA encoding lysosomal enzyme α-galactosidase A [36]. This deficient enzyme function causes accrual of intracellular globotriosylceramide that interferes with cellular metabolism. Besides neurological along with Cardiovascular (CVS) correlation the kidneys might get influenced by proteinuria along with renal failure .Replacement of enzyme treatment remains the most important therapy for avoidance of renal propogation.
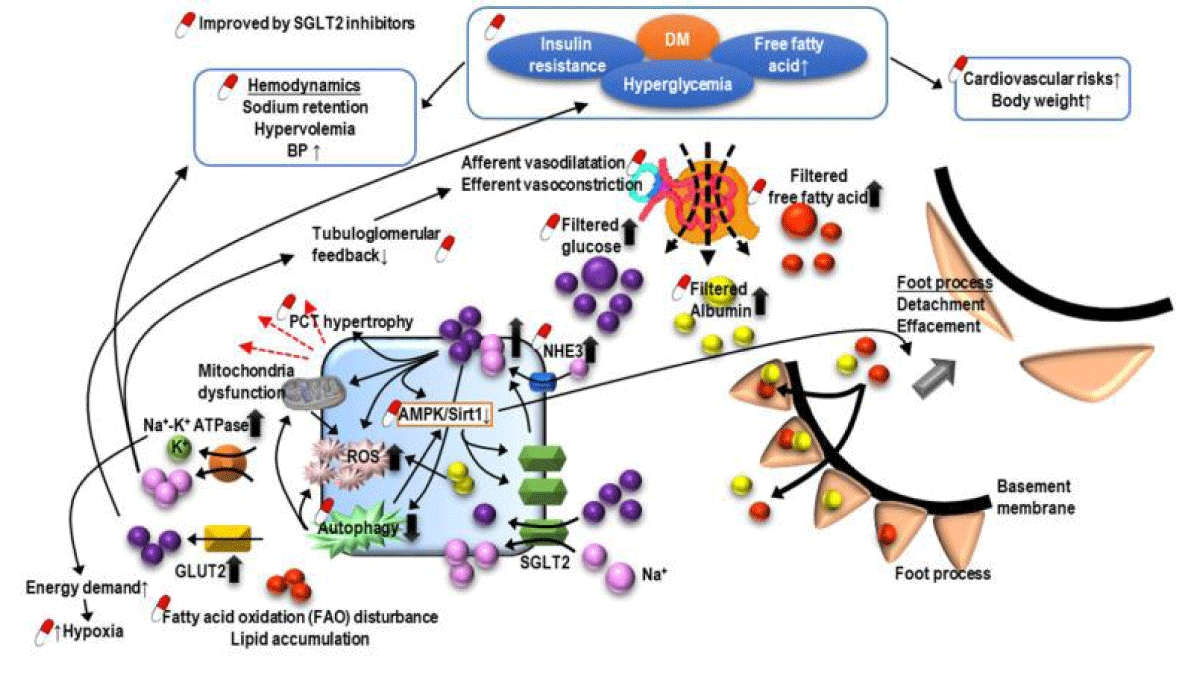
DM correlated CKD: Diabetes mellitus (DM) comprises the commonest causation of CKD along with ESRD all over the world [37]. DKD mostly takes place in patients having had bad glycaemic regulation, however in 30% - 40% it does occur once aggressive glycaemic regulation is there that pointed to be complicated in addition to numerous factors implicated in pathogenesis of DKD [38]. Clinically DKD manifestation is inclusive of dysregulated renal function correlated with proteinuria. The different risk factors observed are inclusive of early initiation of DM, hypertension, obesity, ethnicity, robustness of proteinuria along with smoking [39]. Besides enough glycaemic regulation, blocking of renin-angiotensin –aldosterone system (RAAS) has a significant role in the context of management of DKD. Pentoxiphylline possesses the capacity of postponement of the starting of dialysis along with have considerable anti proteinuric action in patients of DM already in receipt of RAAS blockade [40]. Despite maximum statins illustrate minimal renoprotection actions [41], fenofibrate that constitutes a peroxisome proliferator activated receptor alpha (PPARα) agonist has been demonstrated to possess anti proteinuric actions in DKD [42]. Thiazolidenediones, a PPARγ agonist might be of benefit with regards to DKD along with salt as well as fluid getting retained in the form of an adverse action [43] which needs to be taken into account. Sodium–glucose cotransporter 2 (SGLT2) inhibitors comprise the latest antihyperglycemic drugs that possess the capacity of reduction of blood glucose with effectiveness by avoidance of glucose reabsorption in the PCT. Apart from CVS advantages SGLT2 inhibitors further delay the renal aggravation along with reduction in the robustness of proteinuria in DM patients [44,45] (Figure 3). It has been posited that SGLT2 inhibitors confer protection to the Kidney via escalation of glycaemic regulation resulting in enhancement of cardiovascular function in addition to reduction of body weight apart from restoration of intra renal along with, extra renal haemodynamics that is inclusive of reduction in blood pressure (BP), facilitation of natriuresis along with, resetting the alterations in tubuloglomerular feedback [46]. Furthermore structurally SGLT2 inhibitors are equipped with the quality of reversing hypertrophy of PCT whose induction takes place secondary to insulin resistance (IR) as well as escalation of sodium along with glucose reabsorption along with reduction In Na+- glucose reabsorption via SGLT2 [68]. With the reversing of PCT hypertrophy in addition to reduction In Na+- glucose reabsorption protection of kidney results from reduction in energy needs that is followed by reduction in inflammation, oxidative stress (OS), fibrosis, growth factor expression. Moreover, 5’ AMP-activated protein kinase (AMPK)/SIRT1 signaling that has been repressed secondary to hyperglycemia might get re stimulated by SGLT2 inhibitors for facilitation of anti-inflammatory hypoxia inducible factor (HIF)-2α along with repression of the expression of proinflammatory HIF -1α [48]. Hyperglycemia causes enhancement of reactive oxygen species (ROS), diacylglyceration as well as advanced glycation end-products (AGE), all of which aid in the dysregulation of autophagic removal of SNAII along with activation of p21 as well as p27. Accrued SNAII along with p21 as well as p27 activation cause G2/M phase cell cycle arrest, a trade mark of EMT along with maladaptive renal tubular regeneration. Utilization of SGLT2 inhibitors might cause correction of autophagic clearing along with hampering pathways secondary to the hyperglycemia products that results in amelioration of the cell cycle arrest- associated Kidney injury. Noticeably the observation has been that this renoprotection might get further utilized for nondiabetic patients with Kidney Disease [49].
Figure 3: The pathogenesis of diabetic nephropathy along with how sodium-glucose cotransporter inhibitor (SGLT2Is) confer protection to Kidneys by. i) blocking reabsorption of glucose in PCTii) delay renal worsening in addition to reduction of robustness of proteinuria in patients with DM.Posit is that - SGLT2Is confer protection. by escalation of regulation of glycemia, enhancement of cardiovascular function as well as restoration of intracellular in addition to extracellular haemodynamics, inclusive of reduction of blood pressure (BP) of cardio. With facilitation of natriuresis, along with re activation. Furthermore, reduction of PC Thypertrophy of tubulo glomerular feedback along with decline in whose induction takes place secondary to insulin resistance (IR) as well as escalation of sodium along with glucose reabsorption along with reduction In Na+- glucose reabsorption via SGLT2 [68]. With the reversing of PCT hypertrophy in addition to reduction In Na+- glucose reabsorption protection of kidney results from reduction in energy needs that is followed by reduction in inflammation, oxidative stress (OS), fibrosis, growth factor expression. Moreover, 5’ AMP-activated protein kinase (AMPK)/SIRT1 signaling that has been repressed secondary to hyperglycemia might get re stimulated by SGLT2 inhibitors for facilitation of anti-inflammatory hypoxia inducible factor (HIF)-2α along with repression of the expression of proinflammatory HIF-1α [69-71]. Hyperglycemia causes enhancement of reactive oxygen species (ROS), diacylglyceration as well as advanced glycation end-products (AGE), all of which aid in the dysregulation of autophagic removal of SNAII along with activation of p21 as well as p27. See text for further details as displayed in Figure 3.
O-GlcNAcylation represents a post-translational modifications of proteins as well as possesses the capacity of control of physiological along with pathological events. The part played by O-GlcNAcylation in DM is in the context of insulin resistance (IR) apart from possessing the capacity of the modulation of glucose toxicity followed by DM complications inclusive of DKD. More recently work has illustrated that O-GlcNAcylation of the cellular proteins like ICIn results in dysregulation of the cellular volume control in variable cell kinds a usual occurrence in case of DM [50]. PCT that is hypertrophic is a classical characteristic of DKD since escalation of filtered glucose causes facilitation of sodium along with glucose reabsorption in PCT. O-GlcNAcylation might result in cell demise of PCT thus aiding in the generation of propagation of DKD via dysregulation of cellular volume control that might be reverted by the reduction of ICIn [51]. Furthermore, the observation was that O-GlcNAcylation in PCT had an association with FAO [52].These observations pointed that manipulating O-GlcNAcylation might work in the form of probable therapeutic target for DKD therapy [53].
Hypertension associated CKD: Reduction of blood pressure (BP) is the most significant approach for management of patients with hypertension associated with CKD [54]. The prior belief pointed that a systolic blood pressure (SBP) objective of < 140 mmHg in patients with CKD along with < 130 mmHg in patients with CKD as well as proteinuria [55] would be acceptable. In case of patients with CKD with proteinuria exaggerated BP regulation has a correlaton with lesser serum creatinine doubling or ESRD. Noticeably, Kidney Disease Improving Global Outcomes (KDIGO) updated that the treatment target of SBP regulation of upto < 120 mm in patients with CKD with hypertension might be more appropriate [56].
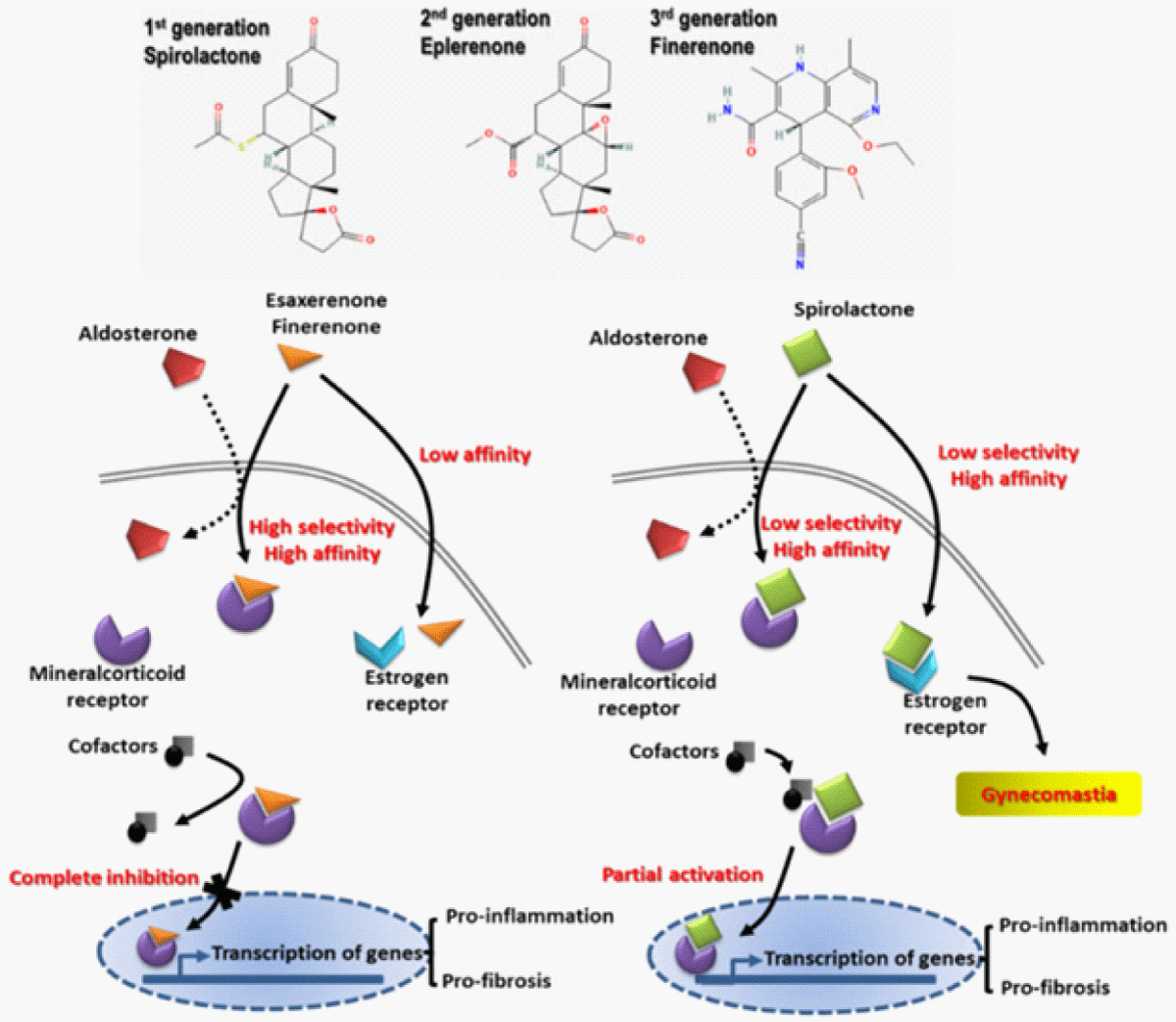
Apart from lifestyle modifications, inclusive of salt reduction or control, stoppage of smoking, reduction of weight, enough exercise, pharmacological treatment can prove to be of advantage with the utilization of blockade of RAAS along with carvediol [57]. Antagonists of mineralocorticoids receptor (MRAs) like spironolactone possess the capacity of reduction of proteinuria as well as BP however are restrictive for CKD treatment in view of correlaton with reduction in GFR as well as hyperkalaemia [58,59]. Innovative nonsteroidal MRAs like esaxerenone along with finerenone got produced, possessing the greater capacity of antifibrotic along with antiinflammatory actions (Figure 4) [60]. In contrast to steroidal MRAs esaxerenone along with finerenone illustrated akin proteinuria reducing actions in patients with CKD along with lesser incidence of hyperkalaemia [61]. Furthermore, combination of single blockade of RAAS with nonsteroidal MRAs demonstrated an akin AKI taking place with finerenone, however it gradually showed reduction in GFR in case of trials with esaxerenone [62]. Nevertheless if combining of nonsteroidal MRAs with other RAAS blocking would actually cause more protection for Kidneys requires more evaluation.
Figure 4: Courtesy ref no-6-The structure of various mineralocorticoids (MRAs). Antagonists of mineralocorticoids receptor like spironolactone possess the capacity of reduction of proteinuria as well as BP however are restrictive for CKD treatment in view of correlaton with reduction in GFR as well as hyperkalaemia [83-85]. Innovative nonsteroidal MRAs like esaxerenone along with finerenone got produced, possessing the greater capacity of antifibrotic along with antiinflammatory actions(In contrast to steroidal MRAs esaxerenone alongwith finerenone illustrated akin proteinuria reducing actions in patients with CKD along with lesser incidence of hyperkalaemia [88-90]. Furthermore, combination of Single blockade of RAAS with nonsteroidal MRAs demonstrated an akin AKI taking place with finerenone, however it gradually showed reduction in GFR in case of trials with esaxerenone MRAs generations such as spironolactone, eplerenone, and novel nonsteroidal MRAs (esaxerenone and finerenone). Novel nonsteroidal MRAs exert better anti-fibrotic and anti-inflammatory effects with renal tubular sparing effects on hyperkalemia.For details see Figure 4 along with text for details.
Heart failure (HF) associated CKD: In patients with CKD, canonical factors do not fully reason out the high prevalence of vascular calcification (VC). This pointed that a CKD- particular pathobiology is implicated in the generation of VC along with escalation of proof points that VC in CKD patients has a unique characteristic of clinical manifestation along with that clinical implications are altered in contrast to those in the general population. More recently Kim, et al. [63], reviewed the mode, diagnostic imaging, as well as clinical characteristic along with what it pointed to as well as strategies of managing VC in patients with CKD [63] (Figures 5-7). Vascular adhesion protein-1 (VAP-1) represents an oxidative enzyme of primary amines that promotes the transmigration of inflammatory cells. Its oxidative and inflammatory actions are prominently escalatied in pathological situations, like metabolic, atherosclerotic, as well as cardiac diseases. Nevertheless, the clinical circulating VAP-1 concentration in hemodialysis (HD) patients is not clear. Thus Kim, et al. [63], in a study of 434 HD patients reported that plasma VAP-1 levels were positively associated with left ventricular diastolic dysfunction as well as the risk of incident cardiovascular and cardiac processes in HD patients. Their outcomes pointed that VAP-1 might help clinicians for the identification of HD patients at a high risk of cardiovascular processes [64]. Trying to evaluate the role of neprilysin as a biomarker in cardiovascular processes in case of haemodialysis (HD) patients, Hwang, et al. [64], observed that greater circulating neprilysin amounts independently anticipated the composite of cardiovascular processes as well as cardiac processes in case of HD patients. The outcomes of this study pointed to the significance of future studies on the actionsof neprilysin hampering in reduction of cardiovascular processes [65]. Neprilysin inhibition (NEPi) as a new therapeutic aapproach with possibility of escalation of results for patients with CKD. NEPi causes the escalation of action of natriuretic peptide systems that resulted in natriuresis, diuresis as well as hampering of the renin–angiotensin system (RAS), that could work as a potentially advantageous counter-regulatory system in states of presence of RAS activation like chronic HF as well as CKD [66]. Furthermore, Judge, et al. [67], demonstrated that NEPi with an angiotensin receptor neprilysin inhibitor (ARNI) [sacubitril/valsartan] might be advantageous in patients with CKD by reduction in the risk of cardiovascular disease (CVD) as well as possessing the potential of retardation of CKD, thus postponement of need for Kidney transplantation [67]. Apart from the canonical medicine for the regulation of heart failure (HF), thus sacubitril that implicates an angiotensin receptor neprilysin inhibitor (ARNI) is advocated at present for reduction of cardiovascular along with hospital admission [68]. Secondary to the common occurrence of dysregulated function in patients with HF the probability of renal benefits of sacubitril/valsartan are undergoing assessment. More recently the observation was that sacubitril/valsartan aided in the reserve renal function along with reduction in the robustness of proteinuria in patients with HF whose presentation was with a reduced ejection fraction (HF rEF) [69]. Jha, et al. [70], in Indian scenario recently successfully treated 2 cases of robust HF with DKD with the utilization of sacubitril/valsartan [70].
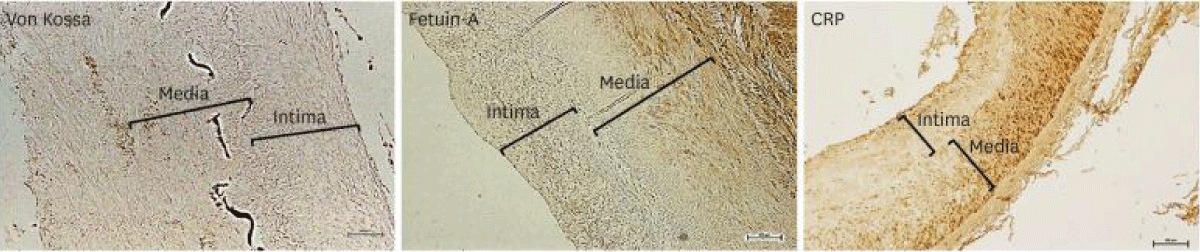
Figure 5: Courtesy ref no-63-Immunohistochemistry of von Kossa, fetuin-A, along with CRP in the iliac artery of kidney transplant recipients. Scale bar 100 μm. Original magnification × 100.39). CRP: C-reactive Protein.
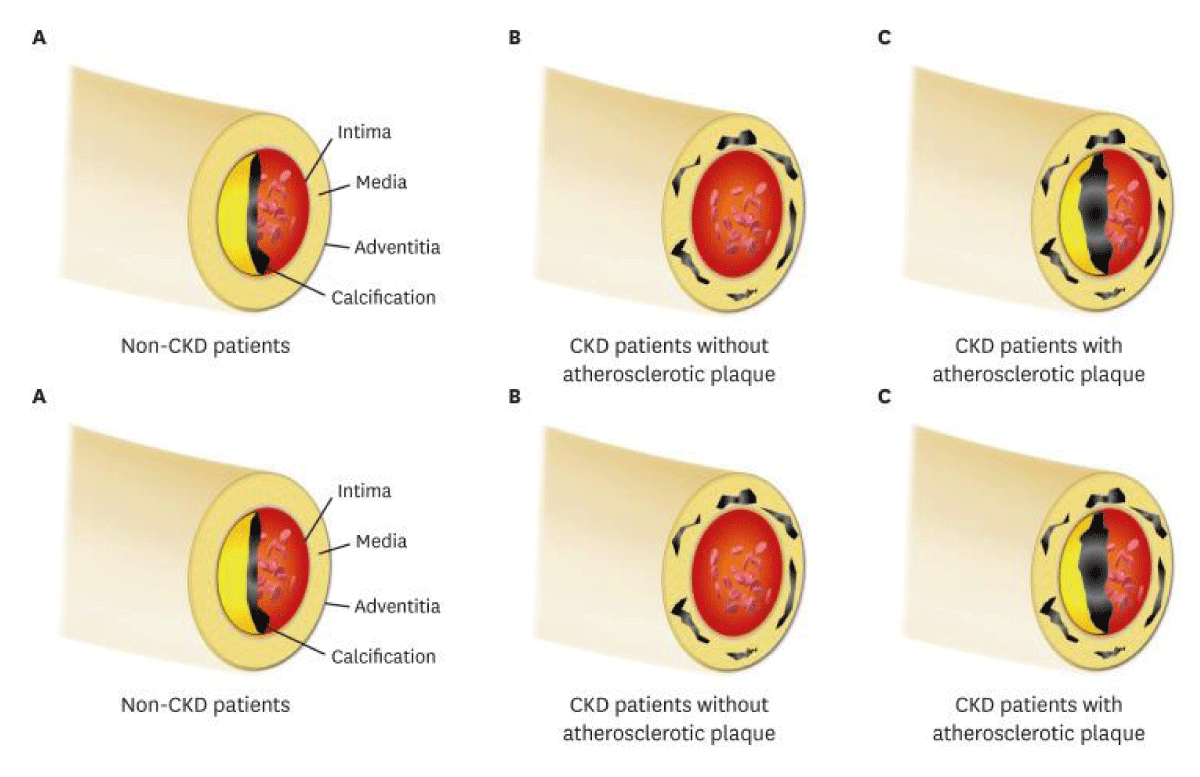
Figure 6: Courtesy ref no-63(A) In patients without renal calcification is placed in the intimal layer; (B) CKD patients derive the medial calcification in the absence of the atherosclerotic factor, in view of mineral bone disorder exaggerates the calcific event; (C) In clinical scenario, intimal along with medial calcification usuall existent together in CKD patients, since they already possessed various traditional risk factors. Noticeably, the intimal or medial calcific burden is higher in CKD patients in contrast to non-CKD patients.
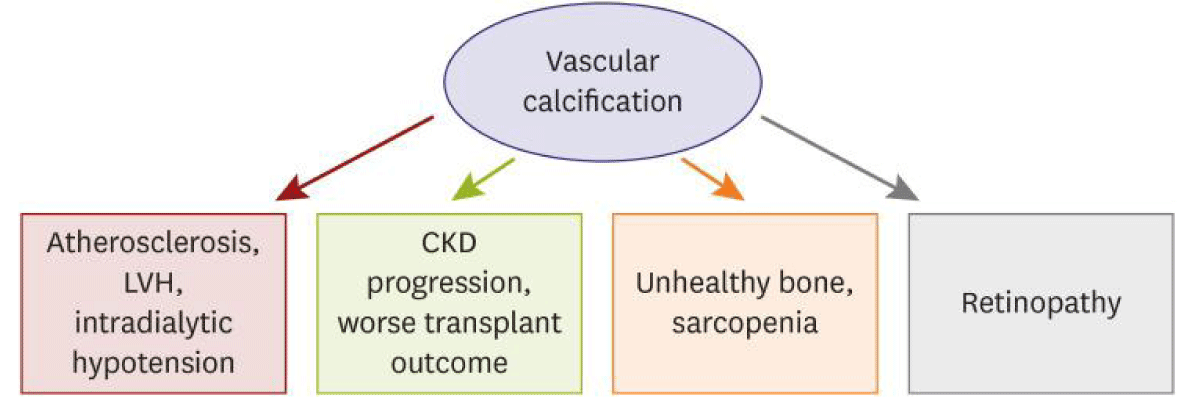
Figure 7: Courtesy ref no-63-Clinical association of vascular calcification to cardiovascular or non-cardiovascular organs in case of CKD. CKD: Chronic Kidney Disease; LVH: Left Ventricular Hypertrophy.
Nephrolithiasis as well as urolithiasis: Nephrolithiasis causes considerable escalation of chances of the incidental presentation of CKD that is responsible for around 2% - 3% of patients with ESRD [71]. Those having production of stone possessed a lesser determined GFR in contrast to the ones without renal calculi [72]. The reason for this is the sharing of numerous risk factors for CKD that is inclusive of the utilization ofnephrotioxic analgesics for the regulation of pain at the time ofuropathy secondary to obstruction, a reduction in consumption of water that results in volume depletion, an escalation of protein consumption, recurrence of sepsis, urinary tract structural aberrations along with contrast media exposure in view of aim of imaging [73]. Noticeably both surgery in addition to shock wave lithotomy result in parenchymal damage, inflammation as well as fibrosis Moreover variability of calculi kinds are associated with variable risks. Like cystine stones possess the maximum risks of propagation of CKD, with uric acid along with struvite calculi [74]. Possessing the knowledge of greater recurrence rate need arises for appropriate management of nephrolithiasis as well as urolithiasis In CKD.
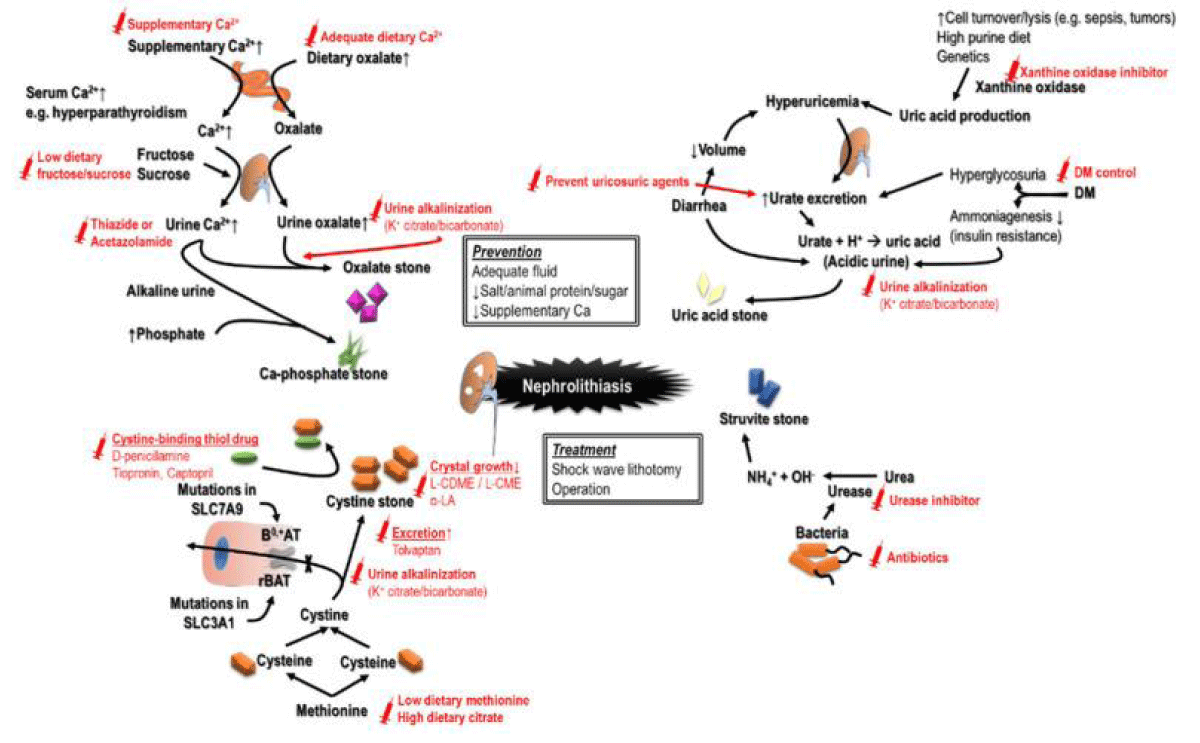
The pathways that result in generation of CKD might be stone particular. Beushite stones commonly produce plugs of the ducts of Bellini that result in duct obstruction, uric acid crystals getting deposited might cause inflammation as well as fibrosis. Chronic pyelonephritis, raised pH of urine secondary to the effect of urease along with staghorn stones in the struvite calculi generators might result in papillary necrosis along with damage to renal parenchyma [75]. Hereditary diseases, not usual might further have manifestation with nephrolithiasis like primary hyperoxaluria, cystinuria, Dent’s disease along with, adenine phosphoribosyl transferase (ARRT) deficiency [76]. The avoidance of generation of stones might be the crucial step for enhancement of results (Figure 8). Much more beneficial renal results have been the observation in uric acid stones in case of prescription of xanthine oxidase inhibitors ; in particular prescription of febuxostat along with sustenance of alkaline urine [77]. However recently Park, et al. [78] in 2022 evinced that allopurinol ameliorated chronic kidney disease propagation along with avoidance of hypouricemia in contrast to febuxostat [78]. Since the therapy might confer renoprotection, further studies on its actions on chronic kidney disease are needed.
Figure 8: Courtesy ref -6-Different causes of nephrolithiasis and urothithiasis with the recommended management strategies to avoid recurrence-i) cause of CKD the utilization ofnephrotioxic analgesics for the regulation of pain at the time ofuropathy secondary to obstruction, a reduction in consumption of water that results in volume depletion, an escalation of protein consumption, recurrence of sepsis, urinary tract structural aberrations along with contrast media exposure in view of aim of imaging [8,97].
Agents particular for stones utilization can be done. Lumasiran an RNAi treatment for type 1 primary hyperoxaluria got approval for clinical utilization recently [79].
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Autosomal Dominant Polycystic Kidney Disease (ADPKD) represents the commonest genetic etiological factor for ESKD [80], besides maximum are carriers of mutations of one of PKD1 or PKD2. More recently GANAB encoding glucosidase IIsubunitα was isolated in the form of a one more pathological gene [81]. Clinical presentation of ADPKD are inclusive of renal (pain in flank, blood in urine, urinary tract infection (UTI), polyuria, nocturia, as well as hypertension), or extra renal (cerebral aneurysm, liver cysts, besides cysts in other organs, valvular heart disease) manifestation [82]. Numerous signaling pathways have been pointed to result in metabolic impairment at the time of natural history of ADPKD, in particular the cAMP pathway that works as the central actor at the time of cystogenesis [83]. Transplantation is thought to be the approach possessing the maximum effectiveness in view of angiotensin converting enzyme inhibitors (ACEi), or angiotensin receptor blocker (ARB) gives provision of restricted action on GFR reduction [84]. Nevertheless, promising outcomes pointed that the utilization of V2 receptor antagonist treatment tolvaptan in patients under the age of 55 yrs possessing an eGFR > 25, l/min/1.73m2 might postpone the deterioration of Kidney function as well as reduction in the volume in a dose based way with enough safety along with tolerance [85].
Autosomal Dominant Tubulointerstitial Kidney Disease (ADTKD)
The properties of Autosomal Dominant Tubulointerstitial Kidney Disease (ADTKD) is tubular injury along with interstitial fibrosis with glomeruli that are not damaged in addition to positive family history [86]. It results in CKD propagation ultimately towards ESRD however is mostly not appreciated despite it being responsible for around 5% of monogenic conditions causing ESRD [87]. The conduction of genetic investigations results in enhancement of sensitivity of the diagnosis. The mutations implicated are having an impact usually in 5 genes, inclusive of UMOD, MUC1, REN, HNFIB SEC61A1. Despite, no particular treatment is existent at present a low salt diet is not advocated along with utilization of diuretics has to get carried out cautiously for avoidance of the exacerbation of salt along with volume depletion along with gout in addition to hyperuricemia [88].
Post transplantation or graft kidney patients
In contrast to dialysis patients in receipt of Kidney transplantation, possess greater advantageous results. Subsequent to induction of treatment with T cell depleting substances, sustenance immunosuppressants that target 3 signals in the context of activation of T cells along with proliferation needs to be persisted with for prevention of rejection [89]. Glucocorticoids are usually employed in the context of induction along with sustenance of immunosuppression via hampering nuclear factor κB (NFκB) along with its downstream expression of cytokines. In case of patients without any direct immune-modulated renal diseases with lesser immunological risk an early stoppage can get taken into account [90]. Antimetabolites are further brought into utilization that is inclusive of azathioprine, mycophenolate/mizoribine in addition to its active metabolites [91].
By blockade ofsignal 1 through binding to the FK506 binding protein (FKBP), Calcineurin inhibitorsI (CNIs) constitute the main medicines. Despite, CNIs resulting in enhancement of graft results at significant rates that is 12 hr trough or 2 h peak (in the context of just neural) serum amounts are required, to get estimated for avoidance of escalated nephrotoxicity [92]. This complicated drug crosstalk of CNIs with the ones possessing the capacity of induction or hampering cytochrome P450 require greater stress. Mammalian target of rapamycin inhibitors (mTORi) further bind to FKBP, however hamper mTOR from blockade of signal 1 [93]. Estimation of proteinuria is required at the time of mTORi for monitoring the adverse actions of denovo proteinuria [94]. The crosstalk of CD28 on T cells for the blockade of signal 2 might get interrupted by betacept binding to CD 80/CD 86 on antigen presenting cells [95]. Patients possessing greater immunological risk ls like the ones with glomeruonephritis in receipt of retransplantation along with the ones having greater high panel–reactive antibody titres are in requirement of sustenance of triple treatment [96]. Subsequent to transplantation having a lesser estimated glomerular filtration rate (eGFR), a mTORi- dependent regimen is believed to be getting preferred more commonly for utilization in contrast to CNIs- dependent regimen [97]. CNIs or antimetabolites might get replaced by mTORi with akin allograft survival, greater post transplant renal function reserve, however in reduction of taking place of non melanoma skin cancers [98]. Other than denovo proteinuria, mTORi might aid in hyperlipidemia, repression of Bone marrow along with infection. Apart from pharmacologic treatment, it is key to carry out continued monitoring of the function of Kidney graft. Whereas serum creatinine escalates it is significant to find reversible factors like sepsis, volume getting depleted along with drug toxicity. Furthermore, BK virus infection is required to get ruled out. Ultrasound aids in assessment of structural changes vascular in flows. In case of recurrent/denovo Kidney diseases or suspicion of rejection, or occurrence of incidental proteinuria Kidney allograft biopsy might be indicated.CKD complications-how to manage
The clinical presentation of CKD are separate as per the causes, m staging along with comorbidities. Apart from handling solute excretion as well as water balance, it further is implicated in sustenance of endocrine homeostasis. With the propagation of CKD uremic toxins accrual takes place. The proper correction of CKD complications might further ameliorate the rate of propagation of CKD.
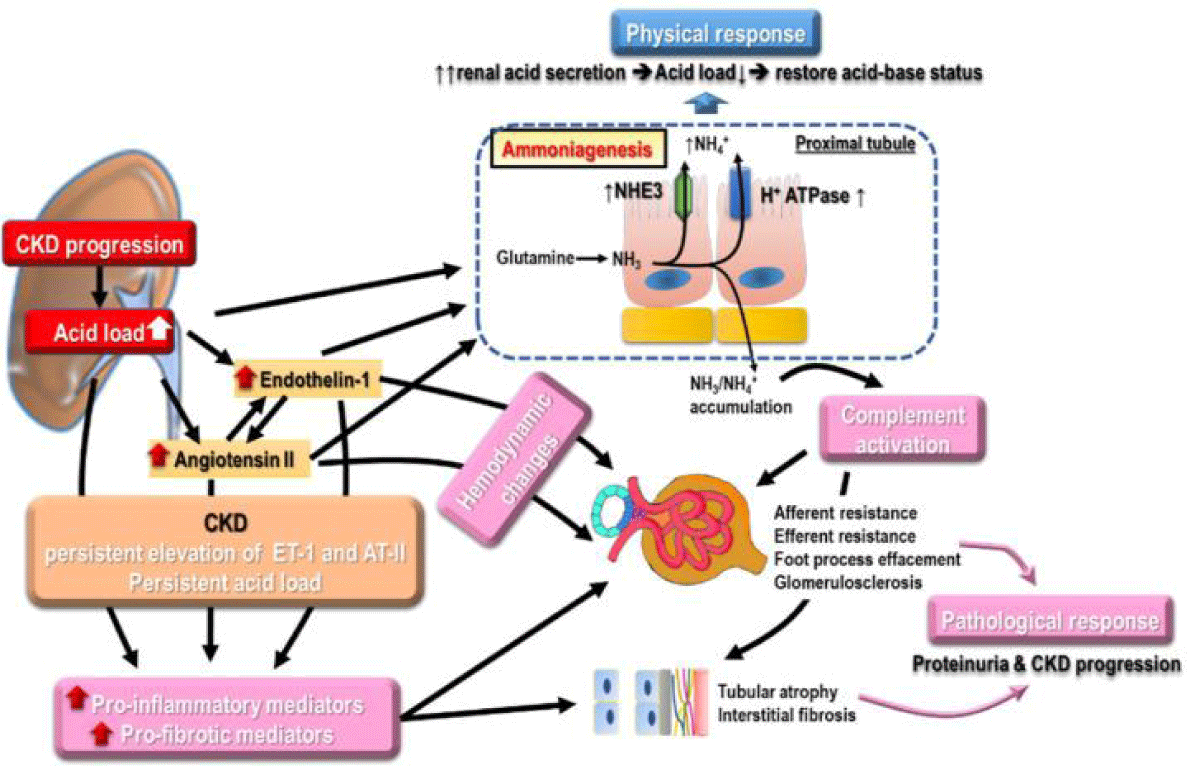
Metabolic acidosis: The continued reduction of renal function along with deteriorated renal ability of excretion of acid along with ammonia production, in addition to escalation of non-volatile acid generation result in metabolic acidosis. Metabolic acidosis incidence was illustrated to cause enhancement in a linear fashion correlated with reduction in GFR [99]. Furthermore metabolic acidosis can aid in propagation of CKD along with utilization of alkali treatment was demonstrated to slow this propagation [100]. The mode of metabolic acidosis stimulated Kidney propagation implicates numerous parameters (Figure 9). Escalation of ammonia generation in the nephrons that are surviving might cause activation of complement correlated with tubulointerstitial injury [101]. Enhancement of endothelin generation associated with metabolic acidosis is observed to cause reduction of GFR along with tubulointerstitial damage [102]. Morethan the renal side actions metabolic acidosis negatively impacts the cardiovascular outcomes (CVOT) through exaggeration of inflammatory reaction, escalation of aldosterone development, enhancement of endothelin generation besides dysregulation of endothelial function, besides reduction in Na+/ K+ ATPase action as well as followed by dyregulation of cardiac contractility [103]. Furthermore metabolic acidosis is correlated with dysregulation of bone mineralization, IR, along with, greater all-cause mortality [104].
Figure 9: Courtesy ref no-6-The numerousmodes of metabolic acidosis on renal propagation like escalation of, ammonia generation in nephrons that escaped damage from complement activationbesides enhancement of endothelin generation, resulting in reduction in GFR and enhancement of tubulointerstitialdamage. Escalation of ammonia generation, in the nephrons that are surviving might cause activation of complement correlated with tubulointerstitial injury [101]. Enhancement of endothelin generation associated with metabolic acidosis is observed to cause, reduction of GFR along with tubulointerstitial damage. More than the renal side actions, metabolic acidosis negatively impacts the cardiovascular outcomes (CVOT) through exaggeration of inflammatory reaction, escalation of aldosterone development, enhancement of endothelin generation besides dysregulation of endothelial function, besides reduction in Na
Early Initiation of alkali treatment might be done for attainment of serum HCO3 concentration amongst 22-26 mEq/l [105], as a HCO3 amount > 26 mEq/l has a correlation with greater mortality as well as cardiac processes [106]. NaHCO3 along with Na citrate are the 2 greater alkalis whose utilization is most usually done, where NaHCO3 supplementation is more economical although might result in bloating, whereas Na citrate is expensive with causing escalation of gastrointestinal absorption of albumin. The botheration in the context of sodium along with volume retention besides hypertension getting aggravated by alkali treatment might get removed with the utilization of diuretics. Fruits besides vegetables have further been illustrated to result in remarkable escalation of serum HCO3- amounts along with aid in preservation of GFR in patients with stage 3 to 4 CKD without the generation of hyperkalaemia, inspite their lesser efficacy in contrast to medicines [107].
Low protein diet consumption with ketoanalogues: High protein consumption results in hyper filteration along with enhancement of intraglomerular pressure causing the origination of propagation of CKD. Hence restricting dietary protein has been believed for a long duration to be of considerable significance in the context of nutritional treatment for CKD. Dietary protein of 0.55 - 0.6 g/kg/day has been pointed for patients with stage 3 to 5 CKD without DM, as well as 0.6 - 0.8 g/kg/day for the ones with DM. In contrast to animal proteins, plant proteins impact glomerular haemodynamics besides lesser net acid generation. The phosphate in proteins of plant origin possess lesser bioavailablity, thus causing lesser phosphate accrual [108]. Plant proteins are correlated with a reduction in the rate of GFR fall clinically with more advantageous result, thus might have the preference as constituting the main source of proteins for patients with CKD [109].
In view of very low protein diet (0.3 - 0.4 g/kg daily protein was documented to cause reduction in risks of incident dialysis in contrast to lesser or normal protein diet, the method of avoidance of protein energy wastage at the time of protein restriction assumes key importance [110]. Ketoanalogues represent the amino acids precursors with their utilization along with simultaneous protein restriction in diet has been seen to cause considerable postponement of CKD besides reduction in the risks of dialysis initiation in patients with (estimated glomerular filtration rate (eGFR) > 18 ml/mln /1.73m2) or without advancement of CKD [111]. Physical exercise in a regular fashion is advocated for patients irrespective of stage of CKD as well as might be of advantage in kidney results. Nevertheless, still there is existence of confusion in the context of the association amongst the protein restriction in diet along with uremic sarcopenia in patients with CKD. Actually physical activity at a regular pattern at the time of a low protein diet or very low protein diet consumption along with ketoanalogues will not result in net protein catabolism, however would aid in enhancement of muscle strength, inflammation as well as nutrition status in case there is existence of enough energy supply [112]. Thus the escalation of nutrition that is free of proteins gives enough provision of energy requirement without the exchange of a high phosphate consumption. Noticeably the utilization of ketoanalogues are efficacious in avoidance of protein - energy wastage, thus might be taken into account as a significant role of nutritional treatment of CKD.
Anaemia: Anaemia is a common complication of CKD correlated with a poor prognosis. The quality of life enhancement occurs in patients with anaemia with CKD, thus correction might result in reduction of the decline in renal function. At present the modes implicated in the generation of renal anaemia is just partly understood [113]. Inadequate erythropoietin (EPO) generation has been pointed to be the main etiology of renal anaemia. Despite introducing erythropoiesis stimulating substances (ESAs) cause reduction of transfusion associated complications besides result in improvement in the symptoms of anaemia [114], no significant enhancement in anaemia associated mortality along with morbidity is seen as the possible reason might be the association of side actions like the deterioration of hypertension, propagation of malignancies, greater cardiovascular complications as well as thrombosis [115]. These observations pointed that the EPO deficit is not just the lone eiological factor for renal anaemia
Hepcidin comprises of a 25 amino acids peptide that gets encoded by HAMP, works in the form of a crucial molecule by reduction of gastrointestinal iron absorption besides results in impaired iron re organization [116]. Interleukin-6 (IL-6) a proinflammatory cytokine results in enhancement of Hepcidin generation, whereas EPO causes escalation of erythroferrone expression for repression of Hepcidin generation [117]. Furthermore, Hepcidin possesses a central part in iron control in patients with CKD either of chronic inflammation or EPO deficit associated with CKD caused escalation of generation of Hepcidin causing dysregulation of iron absorption as well as mobilization of iron from its storage regions [118].
More recently hypoxia inducible factor 1 (HIF) stabilizing substances, like roxadustat as well as vadadustat have got generated for the treatment of renal anaemia [119]. Binding of HIF unlike ESAs to particular sequences that are referred to as hypoxia responsive element (HREs) causes enhancement of the formation of endogenous EPO apart from its utilization in the existence of hypoxia. During normoxia, the hydroxylation of by the propyl hydroxylase domain (PHD) enzymes would cause breakdown of HIF with the idea of control of HIF actions [120]. HIF stabilizing substances have got illustrated to be of non inferiority to ESAs, in particular in non dialysis dependent CKD, in the context of anaemia treatment effectiveness [121]. Nevertheless, with the possession of pleiotropic actions besides long time complications they need greater assessment.
Hyperkalaemia: Hyperkalaemia represents a disorder having the probability of impacting life that is associated with a fast deterioration in renal function in patients with CKD. For escalation of the patients awareness with regards to the significance of restricting dietary potassium (K+) remains the mainstay of management. Despite cationic exchange resins that are inclusive of sodium or calcium polystyrene sulfonate have been utilized broadly in patients with CKD, the correlated sodium load, GIT irritation, incomplete selectiveness in the context of some cations along with their restricted therapeutic effectiveness, cause reduction in their potential use of these resins. More recently innovative substances that are inclusive of zinconium cyclosilicate (ZS-9) as well as Patiromer have got demonstrated to be possessing greater selectivity for potassium, besides milder GIT side actions. ZS-9 might possess a remarkable part in the context of management of acute hyperkalaemia in view of them working fast (within an hour), whereas Patiromer demonstrates a continuous K+ reducing action for 48 h [122]. Significantly the greater effectiveness, of ZS-9 as well as patiromer for reducing K+ might cause enhancement of prognosis of patients with CKD by reduction in the possibility of omission of RAAS blocking that causes a greater advantageous heart with renal results.
Particular situations of CKD
Innovative therapeutic strategies: The restricted actions of the present therapies of CKD cause stimulation of the requirement of Innovative therapeutic substances. Irrespective of the causes of different events, inclusive of fibrosis, inflammation, as well as Oxidative stress (OS) besides dysregulation of cell regeneration are continuing in the Kidney. Evaluation of certain innovative substances that target these pathways was carried out in preclinical studies as well as clinical trials. Like Atrasentan that targets the endothelin-1 receptor ETA is successful in postponement of the propagation of DKD in clinical trials [123]. Having the knowledge of the key part of TGF-β1 in fibrosis in addition to inflammation, oxidation along with apoptosis, pirfenidone (possessing the capacity of reduction of TGF-β1 formation) was illustrated to halt the propagation of estimated glomerular filtration rate (eGFR) in focal segmental glomerulosclerosis (FSGS) clinical trials [124]. MicroRNAs possessing the capacity of controlling different biological events by suppression of translational or modulation of breakdown of mRNA. MiR21 induction is feasible by TGF-β1 in Kidneys, besides is correlated with fibrosis along with podocyte damage. Lamdemirsen,a blocker of miR21,was observed to cause avoidance of the propagation of Alprotsyndrome in preclinical studies [125]. The bromodomain as well as extra terminal (BET) proteins represent epigenetic controllers are implicated in cell proliferation, differentiation along with inflammation. Apabetalone, a BET inhibitor demonstrated promising results in patients with coronary artery disease (CAD) as well as DKD [126,127]. Moreover clinical trials with the utilization of inhibitor of Nrf2 along with p53 are continuing [128].
With the insight, of complicated pathogenesis of CKD that implicates various cell kinds besides alterations of numerous signaling pathways, multi target drugs (MTD) might prove to be a useful approach, like soluble epoxide hydrolase (SHE) dependent PTUPB (with cyclooxygenase2 (COX2) inhibitor), PB394 (with PPARγ agonist) as well as DM509 (with farsenoid X receptor agonist) for amelioration of fibrosis, inflammatory reaction, as well as oxidative stress (OS) from CKD associated with DM, hypertension, hyperlipidemia, or other causes [129].
Mesenchymal stem cells as well as the media that is conditioned: Mesenchymal stem cells (MSCs) possess the properties of capacity of self renewal as well as differentiation to separate cells that can get identified in numerous tissues like adipose tissue (AT) along with Bone marrow [130]. In view of their lower immunogenicity, their transplantation is believed to be a safe therapy, thus its utilization has been done for multiple diseases inclusive of Kidney diseases. MSCs that get transplanted possess the capacity of migrating to the damaged tissue known as MSCs homing that cause immunomodulation, antiapoptosis, antiinflammation along with antioxidation for escalation of tissue healing in the fashion of direct cell-cell crosstalk, or paracrine [131]. Furthermore, ECVs liberated by MSCs as well as the media that is conditioned (CM) of MSCs, MSCs- ECVs or MSCs- CM have been documented to aid in the healing of damaged tissues [132]. In variable models of AKI as well as CKD, the systemic/local injection of MSCs, MSCs- ECVs or MSCs- CM has been illustrated to cause escalation of tubular healing, mitigate inflammation, abrogate fibrosis along with conserve renal function. In case of clinical trials of AKI along with CKD, it was revealed that these were safe in addition to tolerable however the case numbers being less besides smaller period of follow up pointed that the effectiveness of MSCs treatment could not be a conclusive one [133]. In the context of DKD delivery of MSCs possessed a tendency of stabilization or recovery of the GFR in case of patients with T2DM repair however hyperglycemia might cause a reduction in the conferring of renal protection by resulting in injury to the MSCs whose avoidance was feasible by coculturing with macrophages or modulation of MSCs with angiotensin converting enzyme (ACE2) [134]. Akin to that uraemic toxins like p-cresol possess the capacity of impairing the function of MSCs which can get rectified by simultaneous utilization of pioglitzone for the restoration of function of MSCs [135].
Thus having reviewed the role of epigenetics modifications in management of KD, ESRD, Role of Vitamin D in Kidney diseases along with FGF 23, megalin-cubilin, klotho generation, then role of Vitamin C in avoidance of AKI, beside the significance of hampering the renin–angiotensin system (RAS), along with good control of BP with a minimum of 2 antihypertensives as well as advantages of utilization of manidipine in hypertension associated with DM, we attempted to study in detail the avoidance of deterioration of other CKDs besides DKD [24,136-139], with details of how to avoid propagation & management of AKD, along with causes of CKD like DM Glomerulopathy, hypertension, heart disease correlated with CKD, Nephrolithiasis & urolithiasis, inherited genetic diseases like autosomal polycystic kidney disease & ADTKD, graft in renal transplant, innovative strategies inclusive of use of mesenchymal stem cells. Management of CKD is a complicated affair in view of its heterogenous nature, besides the lack of appreciation of its influence of side actions. Other than the lifestyle manipulation, along with the prompt rectification of risk factors for CKD early recognition of AKI in addition to early implementation of medication with regards to managing complications like metabolic acidosis, anaemia, hyperkalaemia as well as CKD-mineral bone disorder (CKD–MBD) are key for slowing the propagation of CKD along with reduction of mortality as well as morbidity. With the greater insight achieved with regards to molecular modes more & more innovatons are getting introduced with their translation will hopefully aid in escalating the outcomes from bench to bedside. Furthermore recently we reported on the role of Vitamin K supplementation in Vitamin K deficiency that is correlated with its presence in case of Chronic Kidney Disease besides in relief provision for vascular calcification & cardiovascular risks for avoidance of mortality [140].
- Kidney Disease ImprovingGlobal Outcomes (KDIGO) CKD Work Group. KDIGO 2012. Clinical Practice Guidelinesfor the evaluation and management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006 Jul;17(7):2034-47. doi: 10.1681/ASN.2005101085. Epub 2006 May 31. PMID: 16738019.
- Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020 May;16(5):269-288. doi: 10.1038/s41581-019-0248-y. Epub 2020 Feb 14. PMID: 32060481.
- Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010 Jul;17(4):293-301. doi: 10.1053/j.ackd.2010.03.010. PMID: 20610356; PMCID: PMC3160131.
- Duan J, Wang C, Liu D, Qiao Y, Pan S, Jiang D, Zhao Z, Liang L, Tian F, Yu P, Zhang Y, Zhao H, Liu Z. Prevalence and risk factors of chronic kidney disease and diabetic kidney disease in Chinese rural residents: a cross-sectional survey. Sci Rep. 2019 Jul 18;9(1):10408. doi: 10.1038/s41598-019-46857-7. PMID: 31320683; PMCID: PMC6639314.
- Yan MT, Chao CT, Lin SH. Chronic Kidney Disease: Strategies to Retard Progression. Int J Mol Sci. 2021 Sep 18;22(18):10084. doi: 10.3390/ijms221810084. PMID: 34576247; PMCID: PMC8470895.
- Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, Toto RD, Wang X, Wright JT Jr, Greene TH. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012 Apr;59(4):504-12. doi: 10.1053/j.ajkd.2011.12.009. Epub 2012 Jan 26. PMID: 22284441; PMCID: PMC3312980..
- Lee DE, Qamar M, Wilke RA. Relative Contribution of Genetic and Environmental Factors in CKD. S D Med. 2021 Jul;74(7):306-309. PMID: 34449991.
- Beyer-Westendorf J, Kreutz R, Posch F, Ay C. The CHA2DS2-VASc score strongly correlates with glomerular filtration rate and predicts renal function decline over time in elderly patients with atrial fibrillation and chronic kidney disease. Int J Cardiol. 2018 Feb 15;253:71-77. doi: 10.1016/j.ijcard.2017.10.110. PMID: 29306476.
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006 Feb;116(2):288-96. doi: 10.1172/JCI27699. PMID: 16453013; PMCID: PMC1359063.
- Ca nades–Garre M, Anderson K, Cappa R, Shelly R, Smyth LJ, et al. Genetic susceptibility to Chronic Kidney Disease -some more pieces for the heritabilitypuzzle. Front Genet. 2019;10:453.
- Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Aspects Med. 2019 Feb;65:16-36. doi: 10.1016/j.mam.2018.06.002. Epub 2018 Jun 22. PMID: 29909119.
- Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M, Rowe RG, Weiss SJ, López-Novoa JM, Nieto MA. Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med. 2015 Sep;21(9):989-97. doi: 10.1038/nm.3901. Epub 2015 Aug 3. Erratum in: Nat Med. 2016 Feb;22(2):217. PMID: 26236989.
- Hajarnis S, Yheskel M, Williams D, Brefort T, Glaudemans B, Debaix H, Baum M, Devuyst O, Patel V. Suppression of microRNA Activity in Kidney Collecting Ducts Induces Partial Loss of Epithelial Phenotype and Renal Fibrosis. J Am Soc Nephrol. 2018 Feb;29(2):518-531. doi: 10.1681/ASN.2017030334. Epub 2017 Oct 11. PMID: 29021386; PMCID: PMC5791084.
- Canaud G, Bonventre JV. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015 Apr;30(4):575-83. doi: 10.1093/ndt/gfu230. Epub 2014 Jul 12. PMID: 25016609; PMCID: PMC4370290.
- Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC, Pentcheva-Hoang T, Nischal H, Allison JP, Zeisberg M, Kalluri R. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med. 2015 Sep;21(9):998-1009. doi: 10.1038/nm.3902. Epub 2015 Aug 3. PMID: 26236991; PMCID: PMC4587560.
- Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY. Impairment of PPARα and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging. J Am Soc Nephrol. 2018 Apr;29(4):1223-1237. doi: 10.1681/ASN.2017070802. Epub 2018 Feb 12. PMID: 29440279; PMCID: PMC5875952.
- Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018 Mar;93(3):568-579. doi: 10.1016/j.kint.2017.09.033. Epub 2018 Jan 17. PMID: 29361307.
- Song Y, Lv S, Wang F, Liu X, Cheng J, Liu S, Wang X, Chen W, Guan G, Liu G, Peng C. Overexpression of BMP‑7 reverses TGF‑β1‑induced epithelial‑mesenchymal transition by attenuating the Wnt3/β‑catenin and TGF-β1/Smad2/3 signaling pathways in HK‑2 cells. Mol Med Rep. 2020 Feb;21(2):833-841. doi: 10.3892/mmr.2019.10875. Epub 2019 Dec 10. PMID: 31974602; PMCID: PMC6947920.
- Tampe B, Tampe D, Nyamsuren G, Klöpper F, Rapp G, Kauffels A, Lorf T, Zeisberg EM, Müller GA, Kalluri R, Hakroush S, Zeisberg M. Pharmacological induction of hypoxia-inducible transcription factor ARNT attenuates chronic kidney failure. J Clin Invest. 2018 Jul 2;128(7):3053-3070. doi: 10.1172/JCI89632. Epub 2018 Jun 11. PMID: 29664738; PMCID: PMC6025987.
- Tampe B, Steinle U, Tampe D, Carstens JL, Korsten P, Zeisberg EM, Müller GA, Kalluri R, Zeisberg M. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int. 2017 Jan;91(1):157-176. doi: 10.1016/j.kint.2016.07.042. Epub 2016 Sep 28. PMID: 27692563.
- Morgado-Pascual JL, Rayego-Mateos S, Tejedor L, Suarez-Alvarez B, Ruiz-Ortega M. Bromodomain and Extraterminal Proteins as Novel Epigenetic Targets for Renal Diseases. Front Pharmacol. 2019 Nov 8;10:1315. doi: 10.3389/fphar.2019.01315. PMID: 31780938; PMCID: PMC6857099.
- Mello MLS. Sodium Valproate-Induced Chromatin Remodeling. Front Cell Dev Biol. 2021 Apr 20;9:645518. doi: 10.3389/fcell.2021.645518. PMID: 33959607; PMCID: PMC8093769.
- Kaur KK, Allahbadia GN, Singh M. Potential role of Epigenetic Modulation in prevention or therapy for Diabetic Kidney Disease-still a dream or a reality–A Systematic Review. J Diab Nephro Diab Mgmt. 2021:1:1(1-26).
- Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, Powe NR, Pavkov ME, Williams DE, Saran R, Shahinian VB; Centers for Disease Control and Prevention CKD Surveillance Team. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2016 May;67(5):742-52. doi: 10.1053/j.ajkd.2015.10.019. Epub 2015 Dec 12. PMID: 26690912; PMCID: PMC6837804.
- Liu KD, Yang J, Tan TC, Glidden DV, Zheng S, Pravoverov L, Hsu CY, Go AS. Risk Factors for Recurrent Acute Kidney Injury in a Large Population-Based Cohort. Am J Kidney Dis. 2019 Feb;73(2):163-173. doi: 10.1053/j.ajkd.2018.08.008. Epub 2018 Oct 25. PMID: 30482577; PMCID: PMC6647831.
- Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009 Nov;76(10):1089-97. doi: 10.1038/ki.2009.332. Epub 2009 Sep 9. PMID: 19741590.
- Kashani K, Kellum JA. Novel biomarkers indicating repair or progression after acute kidney injury. Curr Opin Nephrol Hypertens. 2015 Jan;24(1):21-7. doi: 10.1097/MNH.0000000000000090. PMID: 25415614.
- Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, Gnewuch C, Graf BM, Gnann W, Banas B, Bein T, Schlitt HJ, Bergler T. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann Surg. 2018 Jun;267(6):1013-1020. doi: 10.1097/SLA.0000000000002485. PMID: 28857811.
- Little MH, Kairath P. Does Renal Repair Recapitulate Kidney Development? J Am Soc Nephrol. 2017 Jan;28(1):34-46. doi: 10.1681/ASN.2016070748. Epub 2016 Oct 26. PMID: 27798243; PMCID: PMC5198297.
- Sato Y, Yanagita M. Immune cells and inflammation in AKI to CKD progression. Am J Physiol Renal Physiol. 2018 Dec 1;315(6):F1501-F1512. doi: 10.1152/ajprenal.00195.2018. Epub 2018 Aug 29. PMID: 30156114.
- Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Hervé F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P; SEPSISPAM Investigators. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014 Apr 24;370(17):1583-93. doi: 10.1056/NEJMoa1312173. Epub 2014 Mar 18. PMID: 24635770.
- Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM; ERICCA Trial Investigators. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015 Oct 8;373(15):1408-17. doi: 10.1056/NEJMoa1413534. Epub 2015 Oct 5. PMID: 26436207.
- Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016 May 14;387(10032):2036-48. doi: 10.1016/S0140-6736(16)00272-5. Epub 2016 Feb 25. PMID: 26921911.
- Friedman DJ, Pollak MR. APOL1 and Kidney Disease: From Genetics to Biology. Annu Rev Physiol. 2020 Feb 10;82:323-342. doi: 10.1146/annurev-physiol-021119-034345. Epub 2019 Nov 11. PMID: 31710572.
- Turkmen K, Baloglu I. Fabry disease: where are we now? Int Urol Nephrol. 2020 Nov;52(11):2113-2122. doi: 10.1007/s11255-020-02546-3. Epub 2020 Jul 13. PMID: 32661622.
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017 Mar 25;389(10075):1238-1252. doi: 10.1016/S0140-6736(16)32064-5. Epub 2016 Nov 23. PMID: 27887750.
- Gnudi L, Coward RJM, Long DA. Diabetic Nephropathy: Perspective on Novel Molecular Mechanisms. Trends Endocrinol Metab. 2016 Nov;27(11):820-830. doi: 10.1016/j.tem.2016.07.002. Epub 2016 Jul 25. PMID: 27470431.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017 Dec 7;12(12):2032-2045. doi: 10.2215/CJN.11491116. Epub 2017 May 18. PMID: 28522654; PMCID: PMC5718284.
- Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, Macía M, del Castillo N, Rivero A, Getino MA, García P, Jarque A, García J. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol. 2015 Jan;26(1):220-9. doi: 10.1681/ASN.2014010012. Epub 2014 Jun 26. PMID: 24970885; PMCID: PMC4279740.
- Vogt L, Bangalore S, Fayyad R, Melamed S, Hovingh GK, DeMicco DA, Waters DD. Atorvastatin Has a Dose-Dependent Beneficial Effect on Kidney Function and Associated Cardiovascular Outcomes: Post Hoc Analysis of 6 Double-Blind Randomized Controlled Trials. J Am Heart Assoc. 2019 May 7;8(9):e010827. doi: 10.1161/JAHA.118.010827. PMID: 31020900; PMCID: PMC6512126.
- Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G; DAIS Investigators. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am J Kidney Dis. 2005 Mar;45(3):485-93. doi: 10.1053/j.ajkd.2004.11.004. PMID: 15754270.
- Sarafidis PA. Thiazolidinediones and diabetic nephropathy: need for a closer examination? J Cardiometab Syndr. 2007 Fall;2(4):297-301. doi: 10.1111/j.1559-4564.2007.07834.x. PMID: 18059215.
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657. doi: 10.1056/NEJMoa1611925. Epub 2017 Jun 12. PMID: 28605608.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295-2306. doi: 10.1056/NEJMoa1811744. Epub 2019 Apr 14. PMID: 30990260.
- Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium-Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes. 2019 Feb;68(2):248-257. doi: 10.2337/dbi18-0007. Erratum in: Diabetes. 2019 May;68(5):1094. PMID: 30665953.
- Tejedor Jorge A. Hemodynamic and renal implications of sodium-glucose cotransporter- 2 inhibitors in type 2 diabetes mellitus. Med Clin (Barc). 2016 Nov;147 Suppl 1:35-43. English, Spanish. doi: 10.1016/S0025-7753(17)30624-3. PMID: 28760224.
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016 Sep 6;134(10):752-72. doi: 10.1161/CIRCULATIONAHA.116.021887. Epub 2016 Jul 28. PMID: 27470878.
- Fernandez-Fernandez B, Sarafidis P, Kanbay M, Navarro-González JF, Soler MJ, Górriz JL, Ortiz A. SGLT2 inhibitors for non-diabetic kidney disease: drugs to treat CKD that also improve glycaemia. Clin Kidney J. 2020 Oct 9;13(5):728-733. doi: 10.1093/ckj/sfaa198. PMID: 33123352; PMCID: PMC7577767.
- Costa R, Remigante A, Civello DA, Bernardinelli E, Szabó Z, Morabito R, Marino A, Sarikas A, Patsch W, Paulmichl M, Janáky T, Miseta A, Nagy T, Dossena S. O-GlcNAcylation Suppresses the Ion Current IClswell by Preventing the Binding of the Protein ICln to α-Integrin. Front Cell Dev Biol. 2020 Nov 19;8:607080. doi: 10.3389/fcell.2020.607080. PMID: 33330510; PMCID: PMC7717961.
- Okada Y, Numata T, Sato-Numata K, Sabirov RZ, Liu H, Mori SI, Morishima S. Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction. Curr Top Membr. 2019;83:205-283. doi: 10.1016/bs.ctm.2019.03.001. Epub 2019 Apr 19. PMID: 31196606.
- Sugahara S, Kume S, Chin-Kanasaki M, Tomita I, Yasuda-Yamahara M, Yamahara K, Takeda N, Osawa N, Yanagita M, Araki SI, Maegawa H. Protein O-GlcNAcylation Is Essential for the Maintenance of Renal Energy Homeostasis and Function via Lipolysis during Fasting and Diabetes. J Am Soc Nephrol. 2019 Jun;30(6):962-978. doi: 10.1681/ASN.2018090950. Epub 2019 May 1. PMID: 31043434; PMCID: PMC6551777.
- Fürst J, Gschwentner M, Ritter M, Bottà G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmüller S, Wöll E, Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 2002 May;444(1-2):1-25. doi: 10.1007/s00424-002-0805-1. Epub 2002 Mar 8. PMID: 11976912.
- Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis. 2019 Jul;74(1):120-131. doi: 10.1053/j.ajkd.2018.12.044. Epub 2019 Mar 19. PMID: 30898362.
- Kidney Disease Improving Global Outcomes (KDIGO) BP Work Group. KDIGO 2021. Clinical Practice Guidelinesfor the management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201-13.
- Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, Rahman M, Sanders PW, Textor SC, Weir MR, Townsend RR. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013 Aug;62(2):201-13. doi: 10.1053/j.ajkd.2013.03.018. Epub 2013 May 16. PMID: 23684145; PMCID: PMC3929429.
- Hsu CN, Tain YL. Gasotransmitters for the Therapeutic Prevention of Hypertension and Kidney Disease. Int J Mol Sci. 2021 Jul 21;22(15):7808. doi: 10.3390/ijms22157808. PMID: 34360574; PMCID: PMC8345973.
- Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, Strippoli GF. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020 Oct 27;10(10):CD007004. doi: 10.1002/14651858.CD007004.pub4. PMID: 33107592; PMCID: PMC8094274.
- Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014 Apr 29;(4):CD007004. doi: 10.1002/14651858.CD007004.pub3. Update in: Cochrane Database Syst Rev. 2020 Oct 27;10:CD007004. PMID: 24782282.
- Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021 Jan 7;42(2):152-161. doi: 10.1093/eurheartj/ehaa736. PMID: 33099609; PMCID: PMC7813624.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020 Dec 3;383(23):2219-2229. doi: 10.1056/NEJMoa2025845. Epub 2020 Oct 23. PMID: 33264825.
- Rico-Mesa JS, White A, Ahmadian-Tehrani A, Anderson AS. Mineralocorticoid Receptor Antagonists: a Comprehensive Review of Finerenone. Curr Cardiol Rep. 2020 Sep 10;22(11):140. doi: 10.1007/s11886-020-01399-7. PMID: 32910349.
- Kim JS, Hwang HS. Vascular Calcification in Chronic Kidney Disease: Distinct Features of Pathogenesis and Clinical Implication. Korean Circ J. 2021 Dec;51(12):961-982. doi: 10.4070/kcj.2021.0995. PMID: 34854578; PMCID: PMC8636761.
- Hwang HS, Kim JS, Kim YG, Lee YH, Lee DY, Ahn SY, Moon JY, Lee SH, Ko GJ, Jeong KH. Circulating Neprilysin Level Predicts the Risk of Cardiovascular Events in Hemodialysis Patients. Front Cardiovasc Med. 2021 Jun 15;8:684297. doi: 10.3389/fcvm.2021.684297. PMID: 34212014; PMCID: PMC8239158.
- Kim DK, Lee YH, Kim JS, Kim YG, Lee SY, Ahn SY, Lee DY, Jeong KH, Lee SH, Hwang HS, Moon JY. Circulating Vascular Adhesion Protein-1 Level Predicts the Risk of Cardiovascular Events and Mortality in Hemodialysis Patients. Front Cardiovasc Med. 2021 Sep 7;8:701079. doi: 10.3389/fcvm.2021.701079. PMID: 34557529; PMCID: PMC8452851.
- Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015 May;30(5):738-43. doi: 10.1093/ndt/gfu269. Epub 2014 Aug 18. PMID: 25140014; PMCID: PMC4425478.
- Judge PK, Haynes R. TaleNeprilysin and Neprilysin inhibition in chronic kidney disease. Curr Opin Nephrol Hypertens. 2021 Jan;30(1):123-130. doi: 10.1097/MNH.0000000000000659. PMID: 33148948.
- Solomon SD, Vaduganathan M, L Claggett B, Packer M, Zile M, Swedberg K, Rouleau J, A Pfeffer M, Desai A, Lund LH, Kober L, Anand I, Sweitzer N, Linssen G, Merkely B, Luis Arango J, Vinereanu D, Chen CH, Senni M, Sibulo A, Boytsov S, Shi V, Rizkala A, Lefkowitz M, McMurray JJV. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation. 2020 Feb 4;141(5):352-361. doi: 10.1161/CIRCULATIONAHA.119.044586. Epub 2019 Nov 17. PMID: 31736342.
- Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018 Jul;6(7):547-554. doi: 10.1016/S2213-8587(18)30100-1. Epub 2018 Apr 13. PMID: 29661699.
- Jha R, Mukku KK, Rakesh AK, Sinha S. Successful Treatment of Severe Heart Failure in Advanced Diabetic Kidney Disease Using Angiotensin-neprilysin Inhibitors (Sacubitril/Valsartan) - Report of Two Cases with Review of Options in Literature. Indian J Nephrol. 2021 Nov-Dec;31(6):587-591. doi: 10.4103/ijn.IJN_298_20. Epub 2021 Nov 13. PMID: 35068771; PMCID: PMC8722547.
- Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis. 2011 Jan;57(1 Suppl 1):A8, e1-526. doi: 10.1053/j.ajkd.2010.10.007. PMID: 21184928.
- Gillen DL, Worcester EM, Coe FL. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int. 2005 Feb;67(2):685-90. doi: 10.1111/j.1523-1755.2005.67128.x. PMID: 15673317.
- Uribarri J. Chronic kidney disease and kidney stones. Curr Opin Nephrol Hypertens. 2020 Mar;29(2):237-242. doi: 10.1097/MNH.0000000000000582. PMID: 31972597.
- Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011 Aug;6(8):2069-75. doi: 10.2215/CJN.10651110. Epub 2011 Jul 22. PMID: 21784825; PMCID: PMC3156433.
- Gambaro G, Favaro S, D'Angelo A. Risk for renal failure in nephrolithiasis. Am J Kidney Dis. 2001 Feb;37(2):233-43. doi: 10.1053/ajkd.2001.21285. PMID: 11157364.
- Hoppe B, Martin-Higueras C. Inherited conditions resulting in nephrolithiasis. Curr Opin Pediatr. 2020 Apr;32(2):273-283. doi: 10.1097/MOP.0000000000000848. PMID: 31789978.
- Tran TVM, Maalouf NM. Uric acid stone disease: lessons from recent human physiologic studies. Curr Opin Nephrol Hypertens. 2020 Jul;29(4):407-413. doi: 10.1097/MNH.0000000000000610. PMID: 32398609.
- Park S, Lee JP, Kim DK, Kim YS, Lim CS. Superior effect of allopurinol compared to febuxostat on the retardation of chronic kidney disease progression. PLoS One. 2022 Feb 28;17(2):e0264627. doi: 10.1371/journal.pone.0264627. PMID: 35226683; PMCID: PMC8884483.
- Garrelfs SF, Frishberg Y, Hulton SA, Koren MJ, O'Riordan WD, Cochat P, Deschênes G, Shasha-Lavsky H, Saland JM, Van't Hoff WG, Fuster DG, Magen D, Moochhala SH, Schalk G, Simkova E, Groothoff JW, Sas DJ, Meliambro KA, Lu J, Sweetser MT, Garg PP, Vaishnaw AK, Gansner JM, McGregor TL, Lieske JC; ILLUMINATE-A Collaborators. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J Med. 2021 Apr 1;384(13):1216-1226. doi: 10.1056/NEJMoa2021712. PMID: 33789010.
- Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, Kasiske BL, Odland D, Pei Y, Perrone RD, Pirson Y, Schrier RW, Torra R, Torres VE, Watnick T, Wheeler DC; Conference Participants. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015 Jul;88(1):17-27. doi: 10.1038/ki.2015.59. Epub 2015 Mar 18. PMID: 25786098; PMCID: PMC4913350.
- Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet MP, Ferec C, Le Meur Y, Torres VE; Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease, Harris PC. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016 Jun 2;98(6):1193-1207. doi: 10.1016/j.ajhg.2016.05.004. PMID: 27259053; PMCID: PMC4908191.
- Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019 Mar 2;393(10174):919-935. doi: 10.1016/S0140-6736(18)32782-X. Epub 2019 Feb 25. PMID: 30819518.
- Kim DY, Park JH. Genetic Mechanisms of ADPKD. Adv Exp Med Biol. 2016;933:13-22. doi: 10.1007/978-981-10-2041-4_2. PMID: 27730431.
- Lanktree MB, Chapman AB. New treatment paradigms for ADPKD: moving towards precision medicine. Nat Rev Nephrol. 2017 Dec;13(12):750-768. doi: 10.1038/nrneph.2017.127. Epub 2017 Oct 9. PMID: 28989174.
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Dandurand A, Ouyang J, Czerwiec FS, Blais JD; TEMPO 4:4 Trial Investigators. Multicenter, open-label, extension trial to evaluate the long-term efficacy and safety of early versus delayed treatment with tolvaptan in autosomal dominant polycystic kidney disease: the TEMPO 4:4 Trial. Nephrol Dial Transplant. 2018 Mar 1;33(3):477-489. doi: 10.1093/ndt/gfx043. PMID: 28379536; PMCID: PMC6019005.
- Bleyer AJ, Kidd K, Živná M, Kmoch S. Autosomal Dominant Tubulointerstitial Kidney Disease. Adv Chronic Kidney Dis. 2017 Mar;24(2):86-93. doi: 10.1053/j.ackd.2016.11.012. PMID: 28284384; PMCID: PMC5488707.
- Devuyst O, Olinger E, Weber S, Eckardt KU, Kmoch S, Rampoldi L, Bleyer AJ. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers. 2019 Sep 5;5(1):60. doi: 10.1038/s41572-019-0109-9. PMID: 31488840.
- Eckardt KU, Alper SL, Antignac C, Bleyer AJ, Chauveau D, Dahan K, Deltas C, Hosking A, Kmoch S, Rampoldi L, Wiesener M, Wolf MT, Devuyst O; Kidney Disease: Improving Global Outcomes. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management--A KDIGO consensus report. Kidney Int. 2015 Oct;88(4):676-83. doi: 10.1038/ki.2015.28. Epub 2015 Mar 4. PMID: 25738250.
- Lim MA, Kohli J, Bloom RD. Immunosuppression for kidney transplantation: Where are we now and where are we going? Transplant Rev (Orlando). 2017 Jan;31(1):10-17. doi: 10.1016/j.trre.2016.10.006. Epub 2016 Oct 11. PMID: 28340885.
- De Lucena DD, Rangel ÉB. Glucocorticoids use in kidney transplant setting. Expert Opin Drug Metab Toxicol. 2018 Oct;14(10):1023-1041. doi: 10.1080/17425255.2018.1530214. Epub 2018 Oct 5. PMID: 30265586.
- Clayton PA, McDonald SP, Chapman JR, Chadban SJ. Mycophenolate versus azathioprine for kidney transplantation: a 15-year follow-up of a randomized trial. Transplantation. 2012 Jul 27;94(2):152-8. doi: 10.1097/TP.0b013e31825475a3. PMID: 22728292.
- Casey MJ, Meier-Kriesche HU. Calcineurin inhibitors in kidney transplantation: friend or foe? Curr Opin Nephrol Hypertens. 2011 Nov;20(6):610-5. doi: 10.1097/MNH.0b013e32834b4343. PMID: 21885969.
- Vanhove T, Annaert P, Kuypers DR. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev. 2016;48(1):88-112. doi: 10.3109/03602532.2016.1151037. Epub 2016 Feb 25. PMID: 26912097.
- Fantus D, Rogers NM, Grahammer F, Huber TB, Thomson AW. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol. 2016 Oct;12(10):587-609. doi: 10.1038/nrneph.2016.108. Epub 2016 Aug 1. PMID: 27477490; PMCID: PMC6553645.
- Noble J, Jouve T, Janbon B, Rostaing L, Malvezzi P. Belatacept in kidney transplantation and its limitations. Expert Rev Clin Immunol. 2019 Apr;15(4):359-367. doi: 10.1080/1744666X.2019.1574570. Epub 2019 Feb 7. PMID: 30676815.
- Wong W, Venetz JP, Tolkoff-Rubin N, Pascual M. 2005 immunosuppressive strategies in kidney transplantation: which role for the calcineurin inhibitors? Transplantation. 2005 Aug 15;80(3):289-96. doi: 10.1097/01.tp.0000168436.76784.45. PMID: 16082321.
- Tedesco-Silva H, Del Carmen Rial M, Cruz Santiago J, Mazzali M, Pacheco-Silva A, Torres R. Optimizing the clinical utility of sirolimus-based immunosuppression for kidney transplantation. Clin Transplant. 2019 Feb;33(2):e13464. doi: 10.1111/ctr.13464. Epub 2019 Jan 2. PMID: 30548896.
- Préterre J, Visentin J, Saint Cricq M, Kaminski H, Del Bello A, Prezelin-Reydit M, Merville P, Kamar N, Couzi L. Comparison of two strategies based on mammalian target of rapamycin inhibitors in secondary prevention of non-melanoma skin cancer after kidney transplantation, a pilot study. Clin Transplant. 2021 Mar;35(3):e14207. doi: 10.1111/ctr.14207. Epub 2021 Jan 20. PMID: 33369772.
- Kuczera P, Ciaston-Mogilska D, Oslizlo B, Hycki A, Wiecek A, Adamczak M. The Prevalence of Metabolic Acidosis in Patients with Different Stages of Chronic Kidney Disease: Single-Centre Study. Kidney Blood Press Res. 2020;45(6):863-872. doi: 10.1159/000508980. Epub 2020 Oct 16. PMID: 33070125.
- Di Iorio BR, Bellasi A, Raphael KL, Santoro D, Aucella F, Garofano L, Ceccarelli M, Di Lullo L, Capolongo G, Di Iorio M, Guastaferro P, Capasso G; UBI Study Group. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol. 2019 Dec;32(6):989-1001. doi: 10.1007/s40620-019-00656-5. Epub 2019 Oct 9. Erratum in: J Nephrol. 2020 Jun;33(3):619-620. PMID: 31598912; PMCID: PMC6821658.
- Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010 Dec;78(11):1128-35. doi: 10.1038/ki.2010.348. Epub 2010 Sep 22. PMID: 20861823.
- Wesson DE, Nathan T, Rose T, Simoni J, Tran RM. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007 Feb;71(3):210-7. doi: 10.1038/sj.ki.5002036. Epub 2006 Dec 13. PMID: 17164833.
- Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009 Apr;24(4):1232-7. doi: 10.1093/ndt/gfn633. Epub 2008 Nov 17. PMID: 19015169; PMCID: PMC2721428.
- Tangri N, Reaven NL, Funk SE, Ferguson TW, Collister D, Mathur V. Metabolic acidosis is associated with increased risk of adverse kidney outcomes and mortality in patients with non-dialysis dependent chronic kidney disease: an observational cohort study. BMC Nephrol. 2021 May 19;22(1):185. doi: 10.1186/s12882-021-02385-z. PMID: 34011303; PMCID: PMC8136202.
- de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009 Sep;20(9):2075-84. doi: 10.1681/ASN.2008111205. Epub 2009 Jul 16. PMID: 19608703; PMCID: PMC2736774.
- Dobre M, Yang W, Pan Q, Appel L, Bellovich K, Chen J, Feldman H, Fischer MJ, Ham LL, Hostetter T, Jaar BG, Kallem RR, Rosas SE, Scialla JJ, Wolf M, Rahman M; CRIC Study Investigators. Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): A report from the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Heart Assoc. 2015 Apr 20;4(4):e001599. doi: 10.1161/JAHA.114.001599. PMID: 25896890; PMCID: PMC4579944.
- Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014 Nov;86(5):1031-8. doi: 10.1038/ki.2014.83. Epub 2014 Apr 2. PMID: 24694986.
- Cupisti A, Kalantar-Zadeh K. Management of natural and added dietary phosphorus burden in kidney disease. Semin Nephrol. 2013 Mar;33(2):180-90. doi: 10.1016/j.semnephrol.2012.12.018. PMID: 23465504; PMCID: PMC5797670.
- Alvirdizadeh S, Yuzbashian E, Mirmiran P, Eghtesadi S, Azizi F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. 2020 Nov 17;21(1):489. doi: 10.1186/s12882-020-02079-y. PMID: 33203389; PMCID: PMC7672990.
- Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev. 2020 Oct 29;10(10):CD001892. doi: 10.1002/14651858.CD001892.pub5. PMID: 33118160; PMCID: PMC8095031.
- Yen CL, Fan PC, Lee CC, Kuo G, Tu KH, Chen JJ, Lee TH, Hsu HH, Tian YC, Chang CH. Advanced Chronic Kidney Disease with Low and Very Low GFR: Can a Low-Protein Diet Supplemented with Ketoanalogues Delay Dialysis? Nutrients. 2020 Oct 31;12(11):3358. doi: 10.3390/nu12113358. PMID: 33142717; PMCID: PMC7694025.
- Cupisti A, Licitra R, Chisari C, Stampacchia G, D'Alessandro C, Galetta F, Rossi B, Barsotti G. Skeletal muscle and nutritional assessment in chronic renal failure patients on a protein-restricted diet. J Intern Med. 2004 Jan;255(1):115-24. doi: 10.1046/j.0954-6820.2003.01245.x. PMID: 14687247.
- Nangaku M, Eckardt KU. Pathogenesis of renal anemia. Semin Nephrol. 2006 Jul;26(4):261-8. doi: 10.1016/j.semnephrol.2006.06.001. PMID: 16949463.
- Macdougall IC. Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin J Am Soc Nephrol. 2008 Jan;3(1):200-7. doi: 10.2215/CJN.03840907. Epub 2007 Dec 12. PMID: 18077782.
- Salamin O, Kuuranne T, Saugy M, Leuenberger N. Erythropoietin as a performance-enhancing drug: Its mechanistic basis, detection, and potential adverse effects. Mol Cell Endocrinol. 2018 Mar 15;464:75-87. doi: 10.1016/j.mce.2017.01.033. Epub 2017 Jan 22. PMID: 28119134.
- Pagani A, Nai A, Silvestri L, Camaschella C. Hepcidin and Anemia: A Tight Relationship. Front Physiol. 2019 Oct 9;10:1294. doi: 10.3389/fphys.2019.01294. PMID: 31649559; PMCID: PMC6794341.
- Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016 May;23(3):189-97. doi: 10.1097/MOH.0000000000000236. PMID: 26886082; PMCID: PMC4993159.
- Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012 Oct;23(10):1631-4. doi: 10.1681/ASN.2011111078. Epub 2012 Aug 30. PMID: 22935483; PMCID: PMC3458456.
- Gupta N, Wish JB. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am J Kidney Dis. 2017 Jun;69(6):815-826. doi: 10.1053/j.ajkd.2016.12.011. Epub 2017 Feb 24. Erratum in: Am J Kidney Dis. 2017 Jun;69(6):869. PMID: 28242135.
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001 Oct 5;107(1):43-54. doi: 10.1016/s0092-8674(01)00507-4. PMID: 11595184.
- Chen N, Qian J, Chen J, Yu X, Mei C, Hao C, Jiang G, Lin H, Zhang X, Zuo L, He Q, Fu P, Li X, Ni D, Hemmerich S, Liu C, Szczech L, Besarab A, Neff TB, Peony Yu KH, Valone FH. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrol Dial Transplant. 2017 Aug 1;32(8):1373-1386. doi: 10.1093/ndt/gfx011. PMID: 28371815; PMCID: PMC5837707.
- Colbert GB, Patel D, Lerma EV. Patiromer for the treatment of hyperkalemia. Expert Rev Clin Pharmacol. 2020 Jun;13(6):563-570. doi: 10.1080/17512433.2020.1774363. Epub 2020 Jun 8. PMID: 32511052.
- Heerspink HJL, Andress DL, Bakris G, Brennan JJ, Correa-Rotter R, Dey J, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray J, Perkovic V, Tobe S, Wigderson M, Parving HH, de Zeeuw D. Rationale and protocol of the Study Of diabetic Nephropathy with AtRasentan (SONAR) trial: A clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018 Jun;20(6):1369-1376. doi: 10.1111/dom.13245. Epub 2018 Mar 9. PMID: 29405626; PMCID: PMC5969254.
- Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007 Sep;2(5):906-13. doi: 10.2215/CJN.01050207. Epub 2007 Aug 16. PMID: 17702727.
- Kashtan C. Multidisciplinary Management of Alport Syndrome: Current Perspectives. J Multidiscip Healthc. 2021 May 21;14:1169-1180. doi: 10.2147/JMDH.S284784. PMID: 34045864; PMCID: PMC8149282.
- Martinez-Moreno JM, Fontecha-Barriuso M, Martin-Sanchez D, Guerrero-Mauvecin J, Goma-Garces E, Fernandez-Fernandez B, Carriazo S, Sanchez-Niño MD, Ramos AM, Ruiz-Ortega M, Ortiz A, Sanz AB. Epigenetic Modifiers as Potential Therapeutic Targets in Diabetic Kidney Disease. Int J Mol Sci. 2020 Jun 9;21(11):4113. doi: 10.3390/ijms21114113. PMID: 32526941; PMCID: PMC7312774.
- Kalantar-Zadeh K, Schwartz GG, Nicholls SJ, Buhr KA, Ginsberg HN, Johansson JO, Kulikowski E, Lebioda K, Toth PP, Wong N, Sweeney M, Ray KK; BETonMACE Investigators. Effect of Apabetalone on Cardiovascular Events in Diabetes, CKD, and Recent Acute Coronary Syndrome: Results from the BETonMACE Randomized Controlled Trial. Clin J Am Soc Nephrol. 2021 May 8;16(5):705-716. doi: 10.2215/CJN.16751020. Epub 2021 Apr 27. PMID: 33906908; PMCID: PMC8259488.
- Nezu M, Suzuki N, Yamamoto M. Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am J Nephrol. 2017;45(6):473-483. doi: 10.1159/000475890. Epub 2017 May 13. PMID: 28502971.
- Imig JD. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol Ther. 2018 Dec;192:1-19. doi: 10.1016/j.pharmthera.2018.06.015. Epub 2018 Jun 30. PMID: 29964123; PMCID: PMC6263841.
- Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5-14. doi: 10.3727/096368910X. PMID: 21396235.
- Kuppe C, Kramann R. Role of mesenchymal stem cells in kidney injury and fibrosis. Curr Opin Nephrol Hypertens. 2016 Jul;25(4):372-7. doi: 10.1097/MNH.0000000000000230. PMID: 27191350.
- Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017 Dec 4;8(1):273. doi: 10.1186/s13287-017-0727-7. PMID: 29202871; PMCID: PMC5713024.
- Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25(5):829-48. doi: 10.3727/096368915X689622. Epub 2015 Sep 29. PMID: 26423725.
- Lee SE, Jang JE, Kim HS, Jung MK, Ko MS, Kim MO, Park HS, Oh W, Choi SJ, Jin HJ, Kim SY, Kim YJ, Kim SW, Kim MK, Sung CO, Pack CG, Lee KU, Koh EH. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp Mol Med. 2019 Jul 9;51(7):1-14. doi: 10.1038/s12276-019-0268-5. PMID: 31285429; PMCID: PMC6802630.
- Yoon YM, Lee JH, Yun CW, Lee SH. Pioglitazone Improves the Function of Human Mesenchymal Stem Cells in Chronic Kidney Disease Patients. Int J Mol Sci. 2019 May 10;20(9):2314. doi: 10.3390/ijms20092314. PMID: 31083336; PMCID: PMC6540009.
- Kaur KK, Allahbadia GN, Singh M. An update in the utilization of N-acetylcysteine & vitamin c for tackling the oxidativestress in acute kidney injury secondary to robustsepsis - A systematic review. J Clini Nephrol. 2022; 6: 001-018. DOI: 10.29328/journal.jcn.1001084.
- Kaur KK, Allahbadia GN, Singh M. Potential role of epigenetic modulation in prevention or therapy for Diabetic kidney disease-still a dream or a reality; keynote presentation;2021; at the 3rd Annual Summit on Diabetes, Obesity and Heartheld during December 10, 2021 in Webinar byAllied Academies and the Editors of Journal of Cardiovascular Medicine andTherapeutics. J Cholesterol Heart Disease & J Diabetology.
- Kaur KK, Allahbadia GN, Singh M. Can Vitamin D Supplementation prevent / delay/ halt the progression of Diabetic nephropathy; A Systematic Review on mechanism of Vitamin D Crosstalk with Vitamin D Receptor, with others like Megalin-Cubilin and Amnioless Complex along with FGF23-Klotho Complex. J Clin Diabetes Obes 2021.
- Kaur KK, Allahbadia GN, Singh M. Optimizing cardiovascular outcome in Type 2 diabetes mellitus with better control of diabetes mellitus with empigliflozin and hypertensionwith renin angiotensin system inhibitors and manidipine preferably of the dihydropyridones. Obes Res Open J. 2020; 7 (1): 13-25. doi: 10.17140/OROJ-7-141.
- Kaur KK, Allahbadia GN, Singh M. An update on etiology of Chronic Kidney Disease with role of Associated Vitamin K deficiency in Prevention of Vascular calcification& cardiovascular risks & avoid mortality: A minireview. Acta Scientific Nutritional Health. 2022;6(4).