More Information
Submitted: December 15, 2021 | Approved: January 31, 2022 | Published: February 01, 2022
How to cite this article: Khalil K, West-Thielke P, Lichvar A, Benedetti E, Okoroike H, et al. Time within therapeutic range: A comparison of three tacrolimus formulations in renal transplant recipients. J Clini Nephrol. 2022; 6: 019-025.
DOI: 10.29328/journal.jcn.1001085
Copyright License: © 2022 Khalil K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: ATN: Acute Tubular Necrosis; ANCOVA: Analysis Of Covariance; AMR, Antibody-Mediated Rejection; BPACR: Biopsy-Proven Acute Cellular Rejection; BPAMR: Biopsy-Proven Antibody-Mediated Rejection; BMI: Body Mass Index; CNI: Calcineurin Inhibitor; DSA: Donor-Specific Antibodies; ESRD: End-Stage Renal Disease; EMR: Electronic Medical Record; eGFR: estimated Glomerular Filtration Rate; IBW: Ideal Body Weight; IQR: Interquartile Range; KDPI: Kidney Donor Profile Index; MFI: Mean Fluorescence Intensity; MDRD-4: Modification Of Diet In Renal Disease; PRA: Panel Reactive Antibody; rATG: rabbit Anti-Thymocyte Globulin; rTRS: renal Transplant Recipients; sCr: serum Creatinine; SD: Standard Deviation; TAC: Tacrolimus; TAC-IR: Tacrolimus Immediate-Release Capsules; TAC-XL: Extended-Release Once-Daily Capsules; TAC-XR: Novel Once Daily Tacrolimus Tablets; TTR: The Time Within The Therapeutic Range; TTR %: Percent Time Within The Therapeutic Range
Time within therapeutic range: A comparison of three tacrolimus formulations in renal transplant recipients
Karen Khalil, PharmD1*, Patricia West-Thielke, PharmD2, Alicia B Lichvar, PharmD, MS3, Enrico Benedetti, MD2, Henry Okoroike, PharmD3 and Shree Patel, PharmD3
1NYU Transplant Institute, USA
2Department of Surgery, University of Illinois Hospital, USA
3Department of Pharmacy, University of Illinois Hospital, USA
*Address for Correspondence: Karen Khalil, PharmD, Clinical Pharmacotherapy Specialist, Abdominal Transplant Service, NYU Langone Health, 560 1st Ave, Third Floor, TH380, New York, NY 10016, Tel: 516-987-1627; Email: [email protected]
Background: Currently there are three available formulations of tacrolimus in the United States; these include immediate-release capsules (TAC-IR), extended-release capsules (TAC-XL), and extended-release tablets (TAC-XR). Previous studies have demonstrated non-inferiority between the three formulations in terms of efficacy. The purpose of this study was to compare three formulations of tacrolimus (TAC) and assess differences in time within the therapeutic range (TTR) and variability in levels.
Results: Renal transplant recipients from January 2013 to October 2017 were retrospectively identified for analysis. Deviation from standard TAC protocol or formulation changes excluded patients. The primary outcome compared percent TTR (TTR %) among 3 TAC formulations over the first 90 days post-transplant. TTR was calculated using the Rosendaal method. Secondary outcomes included differences in TAC levels, TAC dose, eGFR, rejection, patient and graft survival between the TAC formulations. TAC-XR demonstrated a significantly higher TTR % compared to TAC-IR and TAC-XL (62.8% vs. 53.3% vs. 60.9%, p = 0.048). In post-hoc analysis, TAC-XR had a higher TTR % compared to TAC-IR (p = 0.065), which approached statistical significance. Average TAC levels, weight-normalized TAC doses, median dose-normalized TAC levels, rejection rates, eGFR, and graft or patient survival were similar among groups.
Conclusion: In the early transplant period, TTR was significantly different among the groups. TAC-XR demonstrated numerically superior time within the therapeutic range. Patient-specific factors such as race, obesity, genetic polymorphisms may impact this variability and clinical outcomes. Further analysis is necessary to understand the effect of each patient-specific factor on TAC exposure.
Tacrolimus is considered a narrow therapeutic index drug due to its pharmacokinetic profile, thus requiring intensive therapeutic drug monitoring. Currently, there are three available formulations of tacrolimus in the United States; these include immediate-release capsules (Prograf, Astellas Pharma, Northbrook, IL, USA; [TAC-IR]), extended-release capsules (Astagraf XL, Astellas Pharma, Northbrook, IL, USA; [TAC-XL]), and extended-release tablets (Envarsus XR, Veloxis Pharmaceuticals, Edison, NJ, USA; [TAC-XR]). TAC XR utilizes MeltDose® technology to create tacrolimus tablets that allow for a slow release of the drug throughout the day with a unique pharmacokinetic profile that has a significantly lower peak [1-3]. Previous studies have demonstrated non-inferiority among the three formulations in terms of efficacy. Comparisons of the three formulations have shown variability in time to peak concentrations as well as dose requirements to achieve similar trough levels and exposure [2,4-7]. Although TAC-XL and TAC-XR are both once-daily formulations, there are key differences in their pharmacokinetics that influence dosing requirements. Tremblay, et al. conducted a study comparing the pharmacokinetic profile of TAC-XR with TAC-IR and TAC-XL and their findings showed a significantly higher exposure on a per milligram basis with TAC-XR [7]. This may in part be due to the MeltDose® technology that enhances the oral bioavailability and controls the release of the drug.
Since cytochrome P3A5 (CYP3A5) is a dominant enzyme in the metabolism of tacrolimus, polymorphisms influencing the expression of this enzyme drastically alter tacrolimus clearance [8]. Previous studies show that individuals that are CYP3A5 expressers (extensive or intermediate metabolizers) have lower dose-adjusted trough blood concentrations compared with CYP3A5 non-expressers (poor metabolizers). CYP3A5 expressers often require higher doses of tacrolimus to achieve therapeutic levels and therefore are potentially at risk for peak-related toxicities [8-10]. Some neurotoxicity side effects are mitigated with TAC-XR as a result of the lower and delayed peak concentration [3,7]. High tacrolimus clearance is also an established risk factor for acute rejection in the early phase after renal transplantation [11,12]. Given its unique pharmacokinetic profile, this formulation has also been shown to decrease intra-day fluctuation. Lower tacrolimus exposure and decreased time within a therapeutic range which can be seen in some cases of intra-day fluctuation have been associated with increased risk for development of de novo donor-specific antibodies (DSA) and acute rejection [7,13,14]. As recently demonstrated by Lichvar, et al., early reduction in DSAs was associated with improved death-censored graft survival in both early and late antibody-mediated rejection [15].
It is well established that sub-therapeutic tacrolimus levels and decreased overall exposure are associated with a higher risk of rejection [11,16-20]. It is imperative to assess differences regarding the time within therapeutic range amongst the three available tacrolimus formulations and the impact on clinical outcomes. Therefore, the purpose of this study is to compare time within the therapeutic range amongst the three available tacrolimus formulations and the correlation to clinical outcomes, especially early acute rejection within the first 90 days. Our hypothesis is that given its unique pharmacokinetic properties and lower intra-day fluctuation, TAC-XR has a higher percent time within the therapeutic range (TTR %) compared to the other TAC formulations in the early post-transplant period.
This was a single-center study at a large academic medical center in a metropolitan area. Prior to the commencement of this study, the University of Illinois Hospital and Health Sciences System Institutional Review Board approved this retrospective review.
All adult renal transplantation recipients between January 2013 to October 2017 were assessed for study inclusion. Patients with planned tacrolimus goals different from standard institutional protocol and multi-organ transplants were excluded. The patients were grouped by tacrolimus formulation.
Demographic data and variables such as tacrolimus levels, tacrolimus doses, graft function, patient and graft survival, and episodes of rejection were collected via chart review. Based on institutional protocol, all high immunologic risk renal transplant recipients (defined as Black race, panel reactive antibody (PRA) > 10%, or re-transplant) received anti-thymocyte globulin (dosed as 1.5 mg/kg/dose of ideal body weight (IBW) for 5 doses). Patients at high risk for acute tubular necrosis (ATN) defined as donor age < 12 years old or > 50 years old, recipient age > 55 years old, cold ischemia time > 24 hours, donor serum creatinine (sCr) > 1.8 mg/dl, donation after cardiac death (DCD), or kidney donor profile index (KDPI) > 85% also received rabbit anti-thymocyte globulin induction. A minority of patients received alemtuzumab induction as per a clinical trial protocol at our institution. Patients at low immunologic risk and low ATN risk received basiliximab (20 mg intravenous infusion on postoperative day 0 and postoperative day 4). Patients were defined as sensitized if PRA > 10%. Donor-specific antibodies were categorized as negative (<700 mean fluorescence intensity (MFI)), weak (700-2000 MFI), moderate (2000-5000 MFI), and strong (> 5000 MFI) based on the institution’s immunology DSA assay.
For maintenance immunosuppression, patients were initiated on TAC-IR (0.1 mg/kg/day based on ideal body weight (IBW) or adjusted body weight (AdjBW); AdjBW = IBW + 0.4 [actual body weight – IBW]). Obese patients categorized as body mass index (BMI) greater than or equal to 30 kg/m2 were dosed based on adjusted body weight. Patients not initiated on an extended-release tacrolimus formulation from the time of transplantation were then converted upon discharge based on patient-specific insurance approval. TAC-IR to TAC-XR or TAC-XL conversions followed the accepted recommendations of 1:1 conversion from TAC-IR to TAC-XL, and approximately 30% dose reduction from TAC-IR to TAC-XR, if tacrolimus levels were at goal [7].
Patients were discharged on tacrolimus and either mycophenolate mofetil (MMF, 2000 mg/day) or enteric-coated mycophenolate sodium (EC-MPS, 1440 mg/day) after a loading-dose period during postoperative days 1 to 4 (2880 mg/day postoperative day 1 and 2160 mg/day post-operative day 2-4). Corticosteroids were tapered down and discontinued by postoperative day 6; our institution followed a steroid-free protocol to mitigate the long-term effects of corticosteroids. Early-corticosteroid withdrawal schedule was as follows (prednisone equivalents): postoperative day 0 (500 mg prior to induction), post-operative day 1 (1 mg/kg/day), postoperative days 2 and 3 (0.5 mg/kg/day), postoperative days 4 and 5 (0.25 mg/kg/day). All corticosteroid doses were calculated using IBW. Corticosteroid therapy was continued in renal transplant recipients who were on chronic corticosteroid therapy prior to transplant or if they had undergone positive crossmatch transplantation given the associated increased risk of rejection.
Goal tacrolimus trough concentrations were 8-12 ng/mL (months 0-2) and then and 5-10 ng/mL thereafter. Time in therapeutic range for tacrolimus trough levels was calculated using Rosendaal’s linear interpolation, which is the gold standard [21]. The Rosendaal method assigns TAC levels to days without measured troughs via a linear plot from the last measured TAC level to the next measured TAC level. Using this linear plot, a value is assigned to each day and then all the measured or assigned values for TAC levels are used to calculate the time within the therapeutic range [21]. Per protocol, tacrolimus trough levels were checked weekly from postoperative day 7 to 84. Most patients were discharged within the first week after transplant, therefore tacrolimus levels were collected beginning Day 7. Any levels determined not to be a trough were excluded from the data analysis; a proper trough was determined through clinical chart review for outpatient levels or timing of the level relative to drug administration if the patient was admitted to the hospital.
Episodes of rejection were classified as empiric rejection, biopsy-proven antibody-mediated rejection (BPAMR), or biopsy-proven acute cellular rejection (BPACR). These were diagnosed either on for-cause or protocol biopsies with criteria as defined in the Banff Classification of Allograft Pathology [22]. Empiric rejection was defined by a > 25% increase in serum creatinine (in the absence of urinary obstruction, dehydration, and/or urinary tract infections) that was presumed to be an acute rejection and treated with high-dose pulse steroids, anti-thymocyte globulin or plasmapheresis and/or intravenous immune globulin in the absence of a renal allograft biopsy. Due to the high frequency of robotic kidney transplants and the intraperitoneal placement of the organ, kidney transplant biopsies were not always done. The decision to forego biopsy was per transplant attending discretion. The primary outcome compared percent TTR (TTR %) among 3 TAC formulations over the first 90 days post-transplant. Secondary outcomes include TAC levels, TAC dose, estimated glomerular filtration rate (eGFR) which was assessed by the Modification of Diet in Renal Disease (MDRD-4) equation, rejection, patient and graft survival. These secondary endpoints were assessed at 1 month, 2 months, and 3 months. The MDRD-4 equation is calculated as GFR (mL/min/1.73 m2) = 175 × (sCr)-1.154 × (Age)-0.203 × (0.742 if female) × (1.212 if Black) [23].
Data were assessed for normality with the Shapiro-Wilk test and were described using descriptive statistics. Non-normally distributed continuous variables were compared with the Mann-Whitney U test (two-way comparison) or the Kruskal-Wallis test (three-way comparison). Parametric continuous data were compared with student’s t - test (2-way comparison) or one-way analysis of variance (ANOVA) (3-way comparison). Post-hoc comparisons of the one-way ANOVA were assessed with Tukey’s method. An Analysis of covariance (ANCOVA) was built to assess TTR % across age (> 65 years vs other), Black race, body mass index (BMI > 40 kg/m2). Statistical analysis was completed using STATA® Version 14 Data Analysis and Statistical Software (StataCorp LP, College Station, TX). All p - values < 0.05 were considered statistically significant.
Demographics data
A total of 187 renal transplant recipients (RTRs) transplanted between January 2013 and October 2017 were reviewed. Of those, 58 patients were excluded primarily for deviation from institution protocol or tacrolimus formulation changes, with a minority having multi-organ transplants. In total, 129 patients were included in this retrospective review. Figure 1 depicts the consort diagram and the number of patients included in each group for analysis. The demographics are summarized in Table 1. There was a significant difference in the presence of DSAs at the time of transplant, which was significantly higher in the TAC-XL group. The majority of patients (75%) underwent polyclonal antibody induction with rabbit anti-thymocyte globulin (rATG), with a minority utilizing alemtuzumab induction (9%). Table 1 describes the cohort demographics.
Figure 1: Consort diagram.
| Table 1: Demographics. | ||||
| TAC-IR (n = 42) | TAC-XL (n = 53) | TAC-XR (n = 34) | p - value | |
| Patients, n (%) | 42 (32.6) | 53 (41.1) | 34 (26.4) | - |
| Average Age, years (SD) | 48.7 (14.8) | 50.3 (13.4) | 49.6 (13.2) | 0.85 |
| Female | 19 (45) | 23 (43) | 18 (53) | 0.67 |
| Race, n (%) Caucasian Black Hispanic Asian Other |
6 (14) 23 (55) 11 (26) 1 (2) 1 (2) |
14 (26) 23 (43) 12 (23) 3 (6) 1 (2) |
4 (12) 22 (65) 8 (23) 0 0 |
0.47 |
| BMI, kg/m2 median (IQR) |
34.3 (23.4 – 43) |
32.6 (28.4 – 39.7) |
29.6 (25.1 – 36.3) |
0.19 |
| Median Class I PRA, % (IQR) | 0 (0 – 8) | 0 (0 – 35) | 0 (0 – 30) | 0.87 |
| Median Class II PRA, % (IQR) | 0 (0 – 20) | 0 (0 – 3) | 0 (0 – 0) | 0.77 |
| DSA at time of transplant, n (%) | 3/15 (20) | 10/17 (59) | 7/27 (26) | 0.04 |
| Induction immunosuppression, n (%) Rabbit anti-thymocyte globulin Basiliximab Alemtuzumab |
32 (76) 7 (17) 3 (7) |
39 (74) 13 (24) 1 (2) |
25 (74) 3 (9) 6 (17) |
0.048 |
| BMI: Body Mass Index; PRA: Panel Reactive Antibody; TAC-IR: Twice-Daily Immediate Release Tacrolimus Capsules; TAC-XL: Once-Daily Extended Release Tacrolimus Capsules; TAC-XR: Once-Daily Tacrolimus Tablets | ||||
Time within therapeutic range data
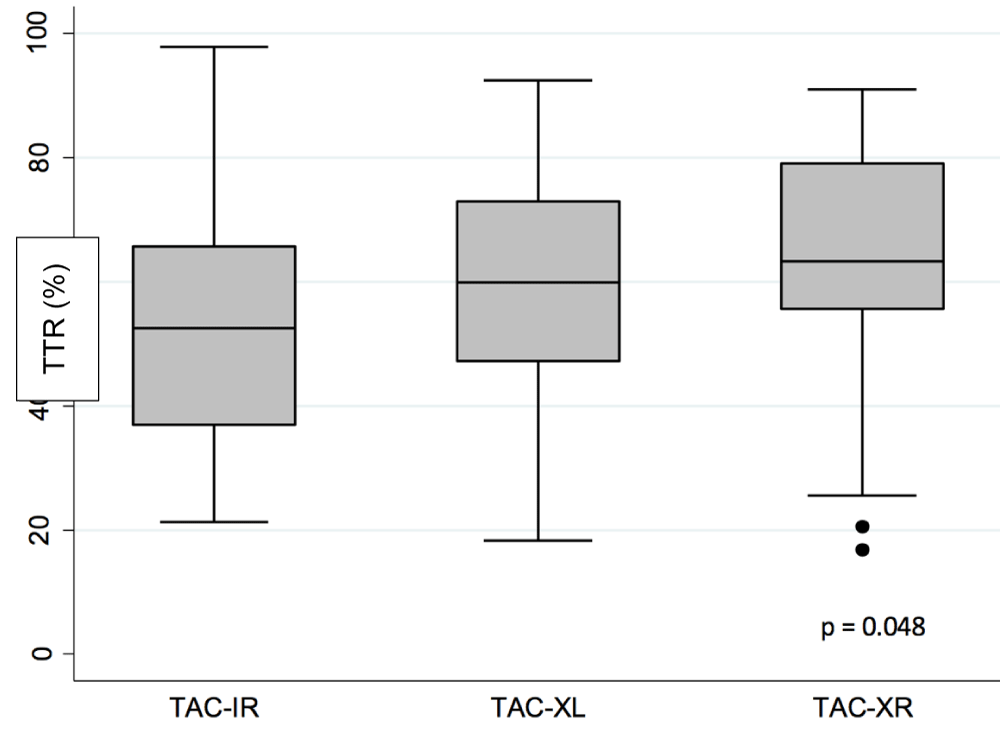
TAC-XR demonstrated a significantly higher TTR % compared to TAC-IR and TAC-XL (TAC-XR 62.8%, TAC-IR 53.3%, TAC-XL 60.9%, p = 0.048) (Figure 2, Table 2). In post-hoc analysis, TAC-XR had a higher TTR % compared to TAC-IR but this result was not statistically significant (p = 0.065).
Figure 2: Time within Therapeutic Range (TTR). TAC-IR: Twice-Daily Immediate Release Tacrolimus Capsules; TAC-XL: Once-Daily Extended Release Tacrolimus Capsules; TAC-XR: Once-Daily Tacrolimus Tablets
Sub-analysis
A sub-analysis was conducted to assess differences in TTR % across gender, age greater than 65 years, obesity, or Black race. There was a significant difference in TTR % when comparing gender (male 55.89% vs. female 62.52%, p = 0.042). There was no difference in TTR % for age, obesity, or Black race. In the ANCOVA, gender (p = 0.029) and TAC formulation (p = 0.032) impacted TTR % significantly when also accounting for Black race, obesity, and age.
Tacrolimus dose data
There was no significant difference in TAC levels, median TAC daily dose, and weight-normalized dose amongst the three formulations (Table 2).
| Table 2: Tacrolimus Dosing Data. | ||||
| TAC-IR (n = 42) | TAC-XL (n = 53) | TAC-XR (n = 34) | p - value | |
| TAC levels, ng/mL (SD) | 8.5 (2.4) | 9.0 (3.7) | 10.7 (4.2) | 0.07 |
| Median TAC daily dose, mg (IQR) | 9 (6 – 15) | 12 (8 – 15) | 10 (7 – 16) | 0.69 |
| Weight-normalized dose, mg/kg (IQR) | 0.15 (0.09 – 0.20) | 0.15 (0.09 – 0.17) | 0.12 (0.7 – 0.16) | 0.59 |
| Median Dose-normalized level, ng/mL/kg (IQR) | 1.21 (0.80 – 1.71) | 1.21 (1.07 – 1.63) | 1.06 (0.57 – 1.52) | 0.59 |
| Tacrolimus TTR, % (SD) | 53.3 (18.7) | 60.9 (16.9) | 62.8 (19.4) | 0.048 |
| TAC-IR: Twice-Daily Immediate-Release Tacrolimus Capsules; TAC-XL: Once-Daily Extended-Release Tacrolimus Capsules; TAC-XR: Once-Daily Tacrolimus Tablets; TAC: Tacrolimus; TTR: Time within the Therapeutic Range | ||||
Rejection data
There was no significant difference in the rates of BPACR, BPAMR, or BPMAR amongst the three groups (Table 3). There was a significant difference in the rate of empiric acute rejection (EAR) amongst the groups with 12% in the TAC-XR group compared to 3% in the TAC-IR group and 0% in the TAC-XL group (p = 0.018). Though there was no statistical difference, the rate of BPACR was 5% in the TAC-IR group and 3% in the TAC-XR group. No patients in the TAC-XL group experienced BPACR. The rate of BPAMR was 0% in the TAC-IR and TAC-XR groups, while 1 patient (2%) in the TAC-XL experienced 1 episode. The rate of BPMAR was 0% in the TAC-IR and TAC-XR group, while 1 patient (2%) experienced 1 episode and another 2 episodes (2%) in the TAC-XL group.
| Table 3: Rejection Data. | ||||
| TAC-IR (n = 42) | TAC-XL (n = 53) | TAC-XR (n = 34) | p - value | |
| BPACR, n (%) No episodes 1 episode |
40 (95) 2 (5) |
53 (100) 0 |
33 (97) 1 (3) |
0.29 |
| BPAMR, n (%) No episodes 1 episode 2 episodes 3 episodes |
42 (100) 0 0 0 |
52 (98) 1 (2) 0 0 |
33 (97) 0 0 1 (3) |
0.37 |
| BPMAR, n (%) No episodes 1 episode 2 episodes |
42 (100) 0 0 |
51 (96) 1 (2) 1 (2) |
33 (97) 0 1 (3) |
0.63 |
| BPACR: Biopsy Proven Acute Cellular Rejection; BPAMR: Biopsy Proven Antibody Mediated Rejection; BPMAR: Biopsy Proven Mixed Acute Rejection; TAC-IR: Twice-Daily Immediate-Release Tacrolimus Capsules; TAC-XL: Once-Daily Extended-Release Tacrolimus Capsules; TAC-XR: Once-Daily Tacrolimus Tablets | ||||
Patient and graft outcomes
There was no difference in graft function, patient and graft survival amongst the three groups (Table 4).
| Table 4: Graft Function and Survival Data | ||||
| TAC-IR (n = 42) |
TAC-XL (n = 53) |
TAC-XR (n = 34) |
p - value | |
| eGFR, mL/min, median (IQR) |
49.5 (43.4 – 63.0) |
41.8 (30.9 – 65.6) |
44.1 (33.5 – 60.6) |
0.34 |
| Allograft survival, n (%) | 42 (100) | 53 (100) | 34 (100) | - |
| Patient survival, n (%) | 42 (100) | 53 (100) | 34 (100) | - |
| eGFR: estimated Glomerular Filtration Rate; TAC-IR: Twice-Daily Immediate-Release Tacrolimus Capsules; TAC-XL: Once-Daily Extended-Release Tacrolimus Capsules; TAC-XR: Once-Daily Tacrolimus Tablets | ||||
To our knowledge, this is the first published study to analyze time within the therapeutic range among the three available tacrolimus formulations and effects on clinical outcomes. There have been a few previous studies comparing pharmacokinetic profiles of the three tacrolimus formulations, proving their non-inferior safety and efficacy [5,7]. We set out to compare the time within the therapeutic range amongst the three available tacrolimus formulations, with a hypothesis that TAC-XR exhibits greater time within the therapeutic range compared to TAC-XL and TAC-IR. In this patient population, TAC-XR demonstrated a significantly higher TTR % compared to TAC-IR and TAC-XL.
The majority of patients included were Black (43% - 65%) in each group and previous studies suggest that the Black race influences tacrolimus concentrations which may contribute to acute rejection or toxicity in RTRs. Taber, et al. found that Black patients were 1.7 times less likely to achieve therapeutic concentrations during the first year after kidney transplant compared to non- Black patients (35% vs. 21%, respectively, p < 0.001). Those that were not therapeutic were 2.4 times more likely to have BPACR and 2.5 times more likely to have BPAMR, as compared with those Black patients achieving therapeutic concentrations [24]. This demonstrates the need to carefully monitor and potentially more aggressively dose Black patients to decrease the risk of rejection. However, our data show no difference in TTR % when comparing Black to non-Black patients, and this variable had no impact on the ANCOVA analysis. The institution protocol calls for rATG induction for high-risk patients, including Black patients, therefore explaining the high rate of rATG induction across the groups. There is limited data on how obesity affects tacrolimus concentrations and variability, however, in our study, this was not significant when compared via t-test or as part of the ANCOVA [25,26]. Given the availability of robotic transplantation at our institution, obese patients, who would have otherwise been disqualified at other centers based on weight, can be transplanted, which explains the mean BMI of the population studied.
TAC-XR had a significantly favorable TTR % compared to TAC-IR and TAC-XL; though this numerical difference needs to be further studied to assess whether it translates to improved clinical outcomes. The observed difference in TTR % may be explained by the unique pharmacokinetics of TAC-XR that allows for a true extended-release formulation that is absorbed throughout the gastrointestinal tract and results in lower intra-day fluctuation, as compared to TAC-XL and TAC-IR [1-3]. The decision was made to utilize TTR % as the primary endpoint rather than coefficient of variation (CV %), which is a ratio of the standard deviation (SD %) to the mean because previous studies have demonstrated the negative effects of tacrolimus level fluctuations on clinical outcomes. Lower tacrolimus exposure has been associated with acute rejection and the development of de novo donor-specific antibodies [17,27]. It is interesting to note the smaller range of variability in the TAC-XR group, which is consistent with previous studies, suggesting lower intra-day fluctuation whether this varies across formulations [7,13]. There were no significant differences in TAC levels, median TAC daily dose, or weight-normalized dose across the groups. This may be in part due to the small sample size. It would be prudent to explore this further in future studies to determine if weight-normalized dose might correlate to clinical outcomes such as rejection and acute calcineurin nephrotoxicity. Knowing this information may help programs better standardize initial dosing.
In terms of rejection outcomes, there was no statistically significant difference in the rates of biopsy-proven rejection across the groups; however, given the limited sample size, it is important to consider numeric differences within the data and how that can guide future more appropriately powered studies to validate these potential differences. There was, however, a significant difference in the rate of empiric acute rejection (EAR) amongst the groups. This may in part be due to protocol for-cause biopsy practice changes based on physician preference and therefore more empiric rejection treatment. Empiric rejection was assessed based on documentation of treatment in the electronic medical record (EMR). Though this was a brief follow-up period of only 3 months, there was 100% graft and patient survival in all groups.
For the sub-analysis, it is interesting to note that though there was only a significant difference in TTR % for gender, the study may not have been powered to detect a difference in obesity and the Black race. Based on known data regarding the phenotypic differences in the Black population that alters tacrolimus metabolism, we would have expected to see a potential difference in TTR % in this sub-population [24,28,29]. Future studies are necessary to determine if there is any significant difference in the TTR % for obesity and Black race and its effects on clinical outcomes such as rejection and graft survival.
A notable limitation previously alluded to is the small sample size which increases the risk of type II error. Though a power analysis was not conducted prior to data analysis, our small sample size may have contributed to an underpowered study therefore unable to detect significant differences if they exist. A larger, multicenter study would be helpful to reevaluate if the increased TTR % found in TAC-XR leads to improved clinical outcomes. Our retrospective design also limits the accuracy and precision of our data based on what is included in the EMR. Adherence was unable to be assessed given the retrospective nature of this study. Inconsistency or documentation errors may have unknowingly confounded our outcomes. The documentation of DSA results in the EMR was inconsistent and practice changes in routinely checking DSAs evolved during the study period. Factors that may have contributed to rejection outcomes such as non-compliance were not assessed. Though our study demonstrated no difference in eGFR between groups, it would be beneficial to expand the study period and assess for tacrolimus concentration correlations to chronic nephrotoxicity and graft function further out from transplantation.
In summary, in the early post-transplant period, TAC-XR demonstrated a higher TTR % compared to the other two formulations within the first 90 days. Further analysis is necessary to understand the effect of patient-specific factors on tacrolimus exposure amongst the different formulations. Future studies are necessary to further explore whether this difference remains consistent and how it impacts clinical outcomes further out from transplant.
Conflict of interest statement: Karen Khalil has received honoria from Veloxis for consulting. Patricia West-Thielke receives grant supports from both Veloxis and Astellas and sits on the Speakers Bureau for Veloxis. All other authors have no conflicts of interest to disclose as described by Journal of Clinical Nephrology.
Authors’ contribution: Drs. Khalil, West-Thielke, Lichvar, Benedetti, Okoroike, and Patel contributed to the design of the manuscript, wrote and revised the manuscript, and gave final approval and are accountable for the information presented.
- Baraldo M. Meltdose Tacrolimus Pharmacokinetics. Transplant Proc. 2016; 48: 420-423. PubMed: https://pubmed.ncbi.nlm.nih.gov/27109969/
- Bunnapradist S, Ciechanowski K, West-Thielke P, Mulgaonkar S, Rostaing L, et al. Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant. 2013; 13: 760-769. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3613750/
- Sanchez Fructuoso A, Ruiz JC, Franco A, Diekmann F, Redondo D, et al. Effectiveness and safety of the conversion to MeltDose((R)) extended-release tacrolimus from other formulations of tacrolimus in stable kidney transplant patients: A retrospective study. Clin Transplant. 2020; 34: e13767. PubMed: https://pubmed.ncbi.nlm.nih.gov/31815310/
- Budde K, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, et al. Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am J Transplant. 2014; 14: 2796-2806. PubMed: https://pubmed.ncbi.nlm.nih.gov/25278376/
- Rostaing L, Bunnapradist S, Grinyo JM, Ciechanowski K, Denny JE, et al. Novel Once-Daily Extended-Release Tacrolimus Versus Twice-Daily Tacrolimus in De Novo Kidney Transplant Recipients: Two-Year Results of Phase 3, Double-Blind, Randomized Trial. Am J Kidney Dis. 2016; 67: 648-659. PubMed: https://pubmed.ncbi.nlm.nih.gov/26717860/
- Silva HT, Jr., Yang HC, Meier-Kriesche HU, Croy R, Holman J, et al. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation. 2014; 97: 636-641. PubMed: https://pubmed.ncbi.nlm.nih.gov/24521771/
- Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. Am J Transplant. 2017; 17: 432-442. PubMed: https://pubmed.ncbi.nlm.nih.gov/27340950/
- Barry A, Levine M. A systematic review of the effect of CYP3A5 genotype on the apparent oral clearance of tacrolimus in renal transplant recipients. Ther Drug Monit. 2010; 32: 708-714. PubMed: https://pubmed.ncbi.nlm.nih.gov/20864901/
- Maldonado AQ, Asempa T, Hudson S, Rebellato LM. Prevalence of CYP3A5 Genomic Variances and Their Impact on Tacrolimus Dosing Requirements among Kidney Transplant Recipients in Eastern North Carolina. Pharmacotherapy. 2017; 37: 1081-1088. PubMed: https://pubmed.ncbi.nlm.nih.gov/28605053/
- Rojas L, Neumann I, Herrero MJ, Boso V, Reig J, et al. Effect of CYP3A5*3 on kidney transplant recipients treated with tacrolimus: a systematic review and meta-analysis of observational studies. Pharmacogenomics J. 2015; 15: 38-48. PubMed: https://pubmed.ncbi.nlm.nih.gov/25201288/
- Baker WL, Steiger S, Martin S, Patel N, Radojevic J, et al. Association Between Time-in-Therapeutic Tacrolimus Range and Early Rejection After Heart Transplant. Pharmacotherapy. 2019; 39: 609-613. PubMed: https://pubmed.ncbi.nlm.nih.gov/30892740/
- Park WY, Paek JH, Jin K, Park SB, Han S. Long-term Clinical Significance of Tacrolimus Trough Level at the Early Period After Kidney Transplantation. Transplant Proc. 2019; 51: 2643-2647. PubMed: https://pubmed.ncbi.nlm.nih.gov/31477420/
- Bunnapradist S, Rostaing L, Alloway RR, West-Thielke P, Denny J, et al. LCPT once-daily extended-release tacrolimus tablets versus twice-daily capsules: a pooled analysis of two phase 3 trials in important de novo and stable kidney transplant recipient subgroups. Transpl Int. 2016; 29: 603-611. PubMed: https://pubmed.ncbi.nlm.nih.gov/26953629/
- Nowicka M, Gorska M, Nowicka Z, Edyko K, Edyko P, et al. Tacrolimus: Influence of the Posttransplant Concentration/Dose Ratio on Kidney Graft Function in a Two-Year Follow-Up. Kidney Blood Press Res. 2019; 44: 1075-1088. PubMed: https://pubmed.ncbi.nlm.nih.gov/31522184/
- Lichvar AB, Tremblay S, Leino AD, Shields AR, Cardi MA, et al. Reducing Donor Specific Antibody During Acute Rejection Diminishes Long Term Renal Allograft Loss: Comparison of Early and Late Rejection. Transplantation. 2020; 104: 2403-2414. PubMed: https://pubmed.ncbi.nlm.nih.gov/32000256/
- Bartlett FE, Carthon CE, Hagopian JC, Horwedel TA, January SE, et al. Tacrolimus Concentration-to-Dose Ratios in Kidney Transplant Recipients and Relationship to Clinical Outcomes. Pharmacotherapy. 2019; 39: 827-836. PubMed: https://pubmed.ncbi.nlm.nih.gov/31230376/
- Davis S, Gralla J, Klem P, Tong S, Wedermyer G, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transplant. 2018; 18: 907-915. PubMed: https://pubmed.ncbi.nlm.nih.gov/28925597/
- Huang CT, Shu KH, Ho HC, Wu MJ. Higher Variability of Tacrolimus Trough Level Increases Risk of Acute Rejection in Kidney Transplant Recipients. Transplant Proc. 2016; 48: 1978-1980. PubMed: https://pubmed.ncbi.nlm.nih.gov/27569931/
- Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014; 85: 1404-1411. PubMed: https://pubmed.ncbi.nlm.nih.gov/24336032/
- Shen CL, Yang AH, Lien TJ, Tarng DC, Yang CY. Tacrolimus Blood Level Fluctuation Predisposes to Coexisting BK Virus Nephropathy and Acute Allograft Rejection. Sci Rep. 2017; 7: 1986. PubMed: https://pubmed.ncbi.nlm.nih.gov/28512328/
- Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69: 236-239. PubMed: https://pubmed.ncbi.nlm.nih.gov/8470047/
- Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018; 102: 1795-1814. PubMed: https://pubmed.ncbi.nlm.nih.gov/30028786/
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145: 247-254. PubMed: https://pubmed.ncbi.nlm.nih.gov/16908915/
- Taber DJ, Gebregziabher MG, Srinivas TR, Chavin KD, Baliga PK, et al. African-American race modifies the influence of tacrolimus concentrations on acute rejection and toxicity in kidney transplant recipients. Pharmacotherapy. 2015; 35: 569-577. PubMed: https://pubmed.ncbi.nlm.nih.gov/26011276/
- Chinnadurai R, Ibrahim ST, Tay T, Bhutani S, Kalra PA. Body weight-based initial dosing of tacrolimus in renal transplantation: Is this an ideal approach? J Ren Care. 2021 47: 51-57 PubMed: https://pubmed.ncbi.nlm.nih.gov/32730692/
- Robert V, Manos-Sampol E, Manson T, Robert T, Decourchelle N, et al. Tacrolimus Exposure in Obese Patients: A Case-Control Study in Kidney Transplantation. Ther Drug Monit. 2021; 43: 229-237 PubMed: https://pubmed.ncbi.nlm.nih.gov/33027230/
- Mendoza Rojas A, Hesselink DA, van Besouw NM, Baan CC, van Gelder T. Impact of low tacrolimus exposure and high tacrolimus intra-patient variability on the development of de novo anti-HLA donor-specific antibodies in kidney transplant recipients. Expert Rev Clin Immunol. 2019; 15: 1323-1331. PubMed: https://pubmed.ncbi.nlm.nih.gov/31721605/
- Narayanan M, Pankewycz O, El-Ghoroury M, Shihab F, Wiland A, et al. Outcomes in African American kidney transplant patients receiving tacrolimus and mycophenolic acid immunosuppression. Transplantation. 2013; 95: 566-572. PubMed: https://pubmed.ncbi.nlm.nih.gov/23423268/
- Trofe-Clark J, Brennan DC, West-Thielke P, Milone MC, Lim MA, et al. Results of ASERTAA, a Randomized Prospective Crossover Pharmacogenetic Study of Immediate-Release Versus Extended-Release Tacrolimus in African American Kidney Transplant Recipients. Am J Kidney Dis. 2018; 71: 315-326. PubMed: https://pubmed.ncbi.nlm.nih.gov/29162334/