More Information
Submitted: December 30, 2021 | Approved: January 27, 2022 | Published: February 01, 2022
How to cite this article: Kaur KK, Allahbadia G, Singh M. An update in the utilization of N-acetyl cysteine & vitamin c for tackling the oxidative stress in acute kidney injury secondary to robust sepsis - A systematic review. J Clini Nephrol. 2022; 6: 001-018.
DOI: 10.29328/journal.jcn.1001084
Copyright License: © 2022 Kaur KK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Sepsis; Acute kidney injury; Oxidative stress; Renal medullary hypoxia; NAC; Vitamin C
An update in the utilization of N-acetyl cysteine & vitamin c for tackling the oxidative stress in acute kidney injury secondary to robust sepsis - A systematic review
Kulvinder Kochar Kaur1* , Gautam Allahbadia2 and Mandeep Singh3
, Gautam Allahbadia2 and Mandeep Singh3
1Dr. Kulvinder Kaur Centre For Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
2Ex-Rotunda-A Centre for Human Reproduction, 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra (W)-400040, Mumbai, India
3Swami Satyanand Hospital, Nawi Kachehri, Baradri, Ladowali Road, Jalandhar, Punjab, India
*Address for Correspondence: Dr. Kulvinder Kochar Kaur, M.D., Scientific Director, Dr. Kulvinder Kaur Centre For Human Reproduction, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India, Email: [email protected]
The commonest etiology of acute kidney injury (AKI) is Sepsis that results in an escalation of morbidity and mortality in the hospital intensive care units. Existentially, the therapy of septic AKI rather than being definitive or curative is just supportive, without tackling the pathophysiology. Usually, Sepsis gets correlated with systemic inflammation, along with the escalated generation of Reactive oxygen species (ROS), in particular superoxide. Simultaneously liberation of nitric oxide (NO) subsequently reacts with the superoxide, thus, resulting in the generation of reactive nitrogen species (RNS), that is mostly peroxynitrite. This sepsis stimulated generation of ROS in addition to RNS might cause a reduction in the bioavailability of NO that modulates microcirculation aberrations, localized tissue hypoxia as well as mitochondrial impairment, thus starting a vicious cycle of cellular damage which results in AKI. Here we conducted a systematic review utilizing search engine PubMed, Google scholar; Web of science; Embase; Cochrane review library utilizing the MeSH terms like septic AKI; ROS; inducible nitric oxide synthase (iNOS); nicotinamide adenine nucleotide phosphate(NADPH)oxidase complex; Oxidative stress; Renal medullary hypoxia; Hypoxia inducible factor1; hypoxia responsive enhancer A; mitochondrial impairment; Intrarenal oxygenation; urinary oxygenation; erythropoietin gene; RRT; NAC; Vitamin C from 1950 to 2021 till date. We found a total of 6500 articles out of which we selected 110 articles for this review. No meta-analysis was done. Thus here we detail the different sources of ROS, at the time of sepsis, besides their pathophysiological crosstalk with the immune system, microcirculation as well as mitochondria that can result in the generation of AKI. Furthermore, we detail the therapeutic utility of N-acetylcysteine (NAC), besides the reasons for its success in ovine as well as porcine models of AKI. Moreover, we discuss preclinical along with clinical for evaluation of Vitamin C’s antioxidant effects as well as pleiotropic effects as a stress hormone that might aid in abrogation of septic AKI.
Sepsis is the commonest etiology of acute kidney injury (AKI) that attributes to about 50% of patients with renal impairment in intensive care units [1]. Generation of AKI at the time of sepsis is important in addition to an independent factor with regards to the prognosis for continuous hospital admission apart from mortality in the hospital [2]. Furthermore, there is escalated epidemiological proof existent in the context of those who survive mild or short periods of AKI possessing greater propensity of generation of Chronic Kidney Disease (CKD) in addition to end stage renal disease (ESRD) in the latter stage of life [3]. Antibiotics along with rejuvenation with fluids besides vasopressors are the existent, recommendations as far as treatment of human sepsis is concerned. Replacement therapy (RRT) is advocated for patients who generate robust septic AKI [4]. Nevertheless, these can maximally aid in alleviation as an intervention measure hoping that patient’s kidney capacity recovers. Considering this getting a good insight into the pathophysiology of septic AKI is the requirement for the generation of efficacious mode-based interventional approaches.
Inspite of global renal ischemia having been posited as the etiology of septic AKI [5], both experimental along with clinical proof questions this posit. Histopathological evaluation conducted on post mortem Kidney tissue from patients dying from septic AKI illustrates heterogeneous focal patchy tubular damage with least tubule-epithelial demise (< 5%), apical vacuolization in addition to the focal mesangial expansion, that are not the properties of robust renal ischaemic damage [6]. Additionally, robust proof from clinically significant ovine along with porcine models is existent concerning sepsis generated AKI occurs despite the lack of global renal ischemia [7]. Akin to the histopathological observation in human sepsis, acute tubular necrosis in addition to tubular cell apoptosis were not the properties of AKI in these large mammalian models of hemodynamic sepsis [7,8]. At the time of inflammatory disorders, like sepsis, there is apparent uncoupling of the renal microcirculation from the macrocirculation [9]. In case of ovine sepsis, particular renal medullary tissue ischemia besides hypoxia antecede the generation of AKI by12-24 h inspite of the escalation of renal blood flow in addition to renal cortical medullary tissue perfusion oxygenation [10,11]. Oxidative stress (OS) possesses a key part in the facilitation of adaptive reaction towards local tissue hypoxia via stabilization of Hypoxia inducible factor 1(HIF 1) that facilitates the transcription of a lot of genes [12]. Nevertheless, in sepsis, an imbalance is existent amongst Reactive oxygen species (ROS) along with Reactive nitrogen species (RNS) as well as the host’s antioxidant defense mode.
Here the sources in addition to part of Oxidative along with nitrosative stress with regards to the pathophysiology of septic AKI highlighting on its cross talk with inflammation, microcirculation anomalies, tissue hypoxia as well as mitochondrial impairment is discussed. More recently, pre-clinical along with clinical studies have evaluated the utilization of antioxidants, mainly N-acetylcysteine (NAC) in addition to Vitamin C in the form of a probable treatment option with regards to septic AKI. Subsequent to conducting a review on the role of Vitamin C for the treatment besides prevention of acute respiratory failure that was inclusive of pneumonia common cold, or any other viral infections like influenza & more specifically in the COVID-19 epidemic we faced all through the world in addition to sepsis [111], we decided to review the role of this antioxidant in therapy of septic AKI.
Here we conducted a systematic review utilizing search engine PubMed, Google Scholar; Web of Science; Embase; Cochrane review library utilizing the MeSH terms like septic AKI; ROS; inducible nitric oxide synthase (iNOS); nicotinamide adenine nucleotide phosphate (NADPH)oxidase complex; Oxidativstress; Renal medullary hypoxia; Hypoxia inducible factor 1; hypoxia responsive enhancer A; mitochondrial impairment; Intrarenal oxygenation; urinary oxygenation; erythropoietin gene; RRT; NAC; Vitamin C from 1950 to 2021 till date.
We found a total of 6500 articles out of which we selected 110 articles for this review. No meta-analysis was done.
Crosstalk amongst the septic inflammatory step way as well as oxidative stress
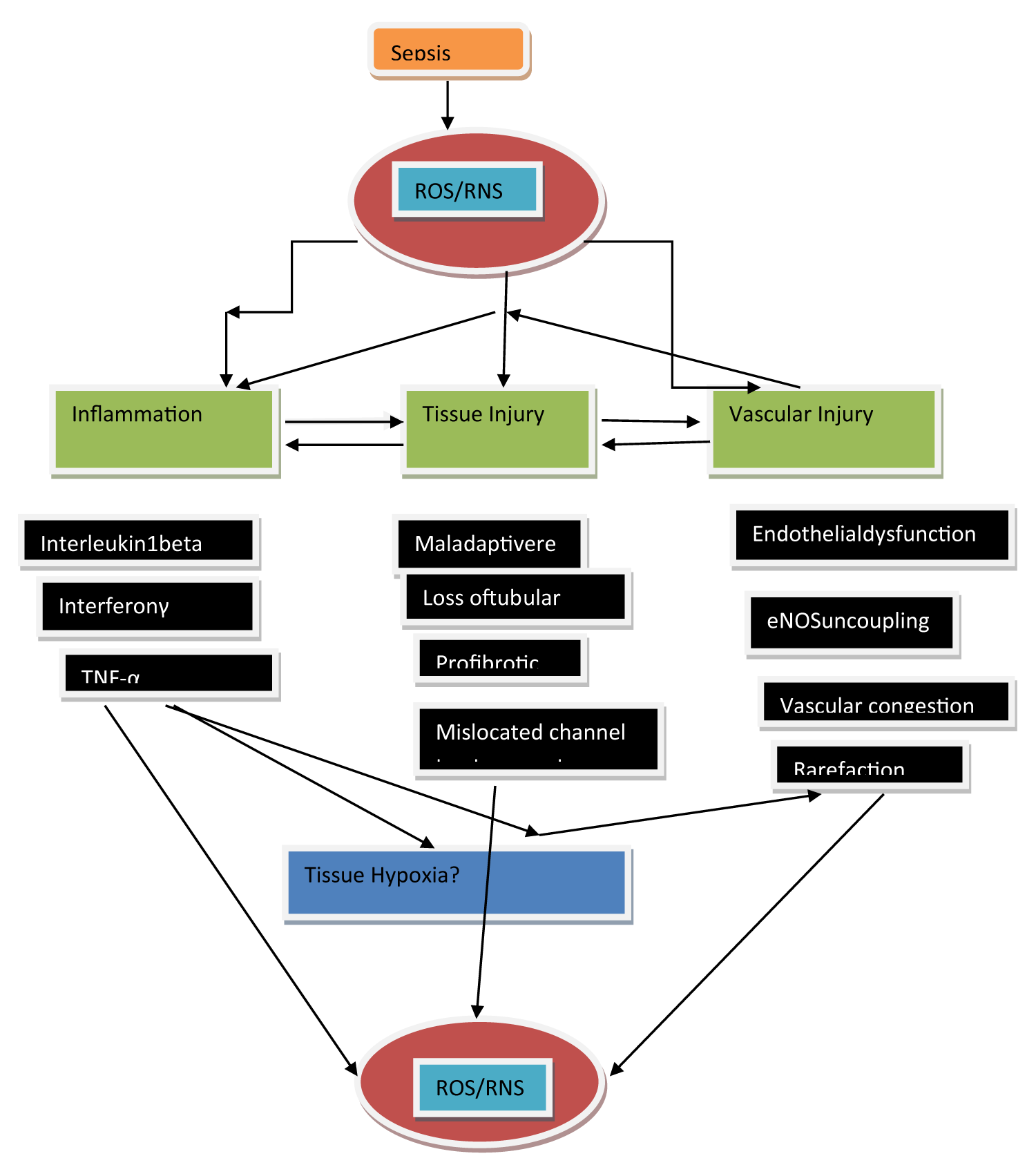
The inflammatory reaction represents the body’s firstline defense from the invader pathogen’s, however, this might work in the form of a key triggering parameter for renal damage. In case of sepsis inflammatory modulators that is inclusive of the damage or pathogen-associated molecular patterns (DAMP/PAMP) get liberated into the intravascular regions, besides getting estimated by toll like receptors (TLR) that are existent on tubular along with endothelial cells [13]. Stimulation of these receptors results in the progression of numerous downstream events that aid in tubular repair, vascular nonrecruitment or temporary perfusion blockade, besides acceleration of proinflammatory immunomodulation at the region of damage that result in vascular congestion along with endothelial impairment [14]. These events seem to come together for the stimulation of superoxide stimulated tissue hypoxia in addition to cellular damage (Figure 1).
Figure 1: Courtesy ref no-15-Scheme of the posited association amongst sepsis- stimulated generation of reactive oxygen species (ROS) along with reactive nitrogen species (RNS) besides inflammation, tissue, in addition to vascular damage. ROS and RNS result in escalated generation of immune-modulatory cells at regions of damage in vessels as well as tubules, thus starting a complex cascade of inflammation along with damage. Significantly, tissue along with vascular damages in robust sepsis can have harmful,actions as they can aid in an uncoupling of endothelial nitric oxide synthase (eNOS), modulated endothelial impairment as well as tissue hypoxia, thereby aid in escalating the accrual of ROS and RNS.
Sepsis stimulated tubular along with vascular in association with Oxidative stress result in the enrolling of polymorphonuclear neutrophils that results in the stimulation of a stepwise immunomodulation process that results in the downstream generation of ROS as well as, RNS that results in the further progression of damage [16]. Neutrophils possess the capacity of developing superoxide via the development of an event that is known as ‘’Oxidative burst’’ [17]. Expression of numerous receptors by neutrophils that are inclusive of β1, β2, as well as β3 integrins. These on activation possess the capacity of binding fibronectin, fibrinogen, besides collagen that causes the modulation of them translocating to extracellular matrix (ECM), by P-selectins as well as E selectins [18]. A number of signaling pathways finally result in the downstream liberation of intracellular calcium along with the generation of nicotinamide adenine nucleotide phosphate (NADPH) oxidase complex that causes the generation of ROS [17]. Moreover, the generation of inducible nitric oxide synthase (iNOS) action gets up regulated in immune cells (Figure 2). The escalation of generation of NO might crosstalk with the ROS that gets generated by NADPH oxidase for the generation of RNS peroxynitrite Figure 2). Peroxynitrite aids in nitrosative injury like S-nitrolysation of proteins thus implicating the normal working of proteins. The thought of ROS stimulated vascular damage is validated by the finding in patients of sepsis of exhaustive generation of superoxide from leukocyte microparticles that further result in an escalation of adhesion molecules action, as well as, endothelial activation [19]. Furthermore, escalated plasma amounts with regards to lipid peroxidation markers (F2-isoprostanes along with isofurans have been robustly correlated with septic AKI patients [20]. The development of oxidative stimuli via neutrophils leads to the luring of proinflammatory chemokine ligand 5 along with intracellular cell adhesion molecule [ICAM]-1, that are significant parameters which promote the enrolment of leukocytes to regions of tissue damage[21]. Leukocytes that have got mobilized besides activated are responsible for stimulation of cytokine storm that implicates the enrolment of proinflammatory cytokines that are inclusive of interleukin-1β (IL-1β), interferon-gamma (IFNγ) as well as (TNFα) [22], that can further cause generation of ROS (Figure 1). Actually, inflammatory, as well as, Oxidative stress biomarkers, like TNFα, IL-1β along with myeloperoxidase action, malondialdehyde (MDA) in addition to hydrogen peroxide (H2O2) have all been documented to get important escalation in patients of septic AKI [23]. Collectively, the original inflammatory step pathway in sepsis seems to be a key parameter responsible for the progression of Oxidative stress that can possess harmful actions concerning renal microcirculation.
Oxidative stress exaggerates microcirculation aberrations besides vascular apparent compressive/blockade action
Endothelial impairment in addition to microcirculation apparent compressive/blockade action alias rarefaction have got documented as the usual pathophysiological characteristics of septic AKI along with are posited to be the key parameters responsible for the modulation of propagation of CKD subsequent to the recovery from AKI [24]. The NO system is a significant controller of the vascular tone amongst renal microcirculation, however, it might be harmfully influenced in sepsis (Figure 2). Amongst the healthy situation, the bio generation of NO by vascular endothelial cells is based on the coupling status of endothelial nitric oxide synthase (eNOS) along with the bioavailability of the cofactor tetrahydrobiopterin (BH4) [25]. Once the endogenous amounts of BH4 are enough, L-arginine coupling occurs with a decrease of oxygen amounts that results in the generation of the robust vasodilator NO [26]. Nevertheless, with low amounts of BH4 there is uncoupling of eNOS with generation of superoxide taking place rather. Moreover, BH4 possess greater proneness towards oxidization to BH2 once greater amounts of superoxide are existent further results in deletion of the pool of the rate limiting cofactor BH4 [27]. Uncoupling of eNOS has been documented to aid in the pathophysiology of several Kidney Diseases that occur secondary to type 2 diabetes mellitus (T2DM), hypertension along with ischemia-reperfusion damage [28]. Intravenous supplementation of BH4 in case of an ovine model of sepsis aids in the rectification of microvascular impairment through the escalation of the amounts of vessels getting perfusion, the percentage of small vessels getting perfusion in addition to the microvascular index amongst the sublingual circulation [29]. In case of an ovine model of robust septic AKI, that got stimulated by the live intravenous infusion of Escherichia Coli, for 48 h eNOS gene expression got selectively downregulated in the renal medulla, however, not in the renal cortex [8]. Nevertheless, if an uncoupling of eNOS aids in the early initiation of microcirculation aberration in the renal medulla in an ovine septic AKI [10] it asks for a greater evaluation.
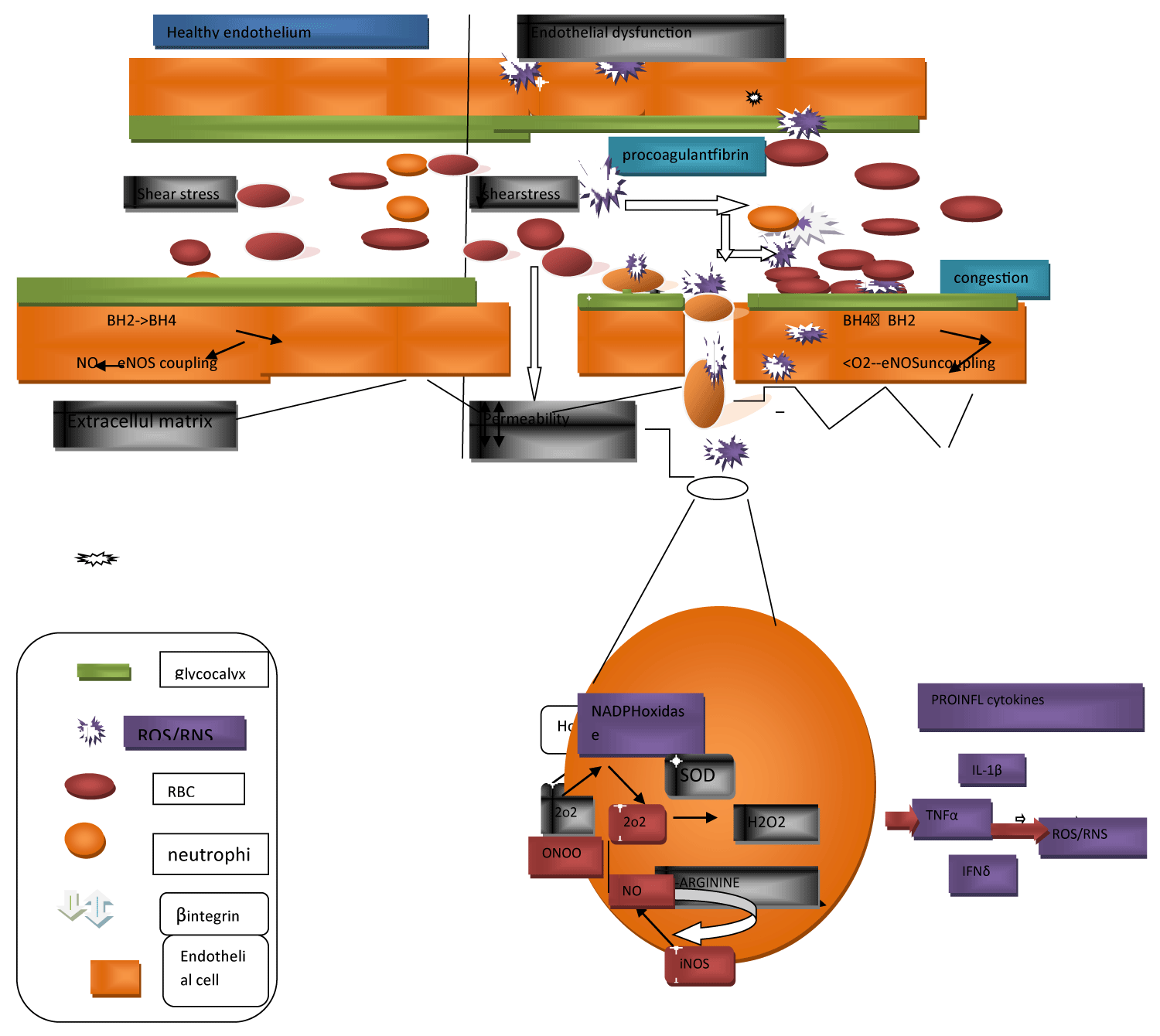
In case of sepsis, escalation of superoxide formation as well as collection in parallel with inflammation further caused a direct structural injury to the vasculature that caused leaking from the vasculature besides tissue edema (Figure 2) [30]. The endothelial glycocalyx is a key controller of endothelial working along with vascular tone. The endothelial glycocalyx that lines the apical surface of the endothelium, is made up of hyaluronic acid, heparin sulfate, glycoproteins along with proteoglycans, that makes sure of the existence of the intactness of vascular permeability, thus it confers protection from the vascular leaking, coagulation, in addition to continuous inflammation (Figure 2) [30]. Further, it is a significant, mechanotransducer in view of its capacity of detection of shear stress, thus aid in shear stress modulated NO generation along with vasodilation. During pathological situations that possess the properties of Oxidative stress, the generation of hyaluronidases which catalyze the depolymerization along with degranulation of the hyaluronic acid of the glycocalyx gets up regulated. The shedding that results, besides the thinning of the endothelial glycocalyx can result in the reduction of the capacity of the endothelium to respond to the shear stress modulated liberation of NO (Figure 2) [31]. Plasma amounts of endothelial glycosaminoglycans in the patients who were the survivors of sepsis were twice in contrast to the healthy volunteers [32]. Furthermore, this casting action of the vascular endothelial cadherin, a protein necessary for the sustenance of gap junctions amongst endothelial cells was importantly higher in case of septic patients with AKI which needed RRT in contrast to the ones without AKI [33]. These clinical findings validate the belief that deletion of endothelial intactness might possess a key part with regards to pushing aberrations in the microcirculation in septic AKI.
Figure 2: Courtesy ref no-15-Schemedetailing the starting of oxidative injury to the endothelium, the downstream consequences for endothelial impairment as well as the progresssion of oxidative stress. The layer of glycocalyx lining the apical surface of endothelial cells is significant for the sustenance of shear-stress and flow- modulated nitric oxide (NO) liberation and vasodilation of the endothelium. Sepsis- stimulated oxidative stress resulted in glycocalyx thinning and shedding, leads to the depletion of gap junctions, along with subsequently escalated permeability impairment as well as vascular leakage. Depletion s of gap junctions further greatly promotes extravasation of neutrophils in the circulation, modulated by β-integrins, via to the extracellular matrix. The resultant superoxide (O2-) that is generated can crosstalk with nitrosative species produced by inducible nitric oxide synthase (iNOS), generating the greatly reactive peroxynitrite (ONOO−) in addition to then hydrogen peroxide (H2O2) subsequently. Oxidative bursts from neutrophilsaid in escalating oxidative injury along with the downstream generation of pro-inflammatory cytokines which have the intrinsic capability of generating reactive oxygen species (ROS) along with reactive nitrogen species (RNS). Oxidative stress can further induce the recruitment of pro-coagulants to the site of injury, causing vascular congestion, ultimately impeding blood flow. Increased oxidative stress at the endothelium depletes besides resulting in oxidation of the pool of tetrahydrobiopterin (BH4), resulting in the uncoupling of endothelial nitric oxide synthase (eNOS) in endothelial cells, thereby enhancing the generation of ROS. Interleukin-1beta (IL-β); tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-δ).
The injured endothelium further lures leukocytes towards the region of damage, as an integral part of the, innate immune response that gets promoted by the exposed intercellular cell along with vascular adhesion molecules. This homing of the proinflammatory cells along with the gap junctions that have got negotiated, results in extravasation of the proinflammatory cells from the endothelium within the tissue that is surrounding, aids in the generation of inflammation [34]. Noticeably inflammatory cells possess the capacity of generation of ROS by themselves in addition to the bioavailability of NO [34], thus aiding the exhaustive pool of superoxide, that necessarily results in the generation of a vicious cycle of Oxidative stress, inflammation along with vascular damage [16] (Figure 1). Microvascular damage that occurs secondary to sepsis stimulation can further cause the liberation of microparticles into the systemic circulation. Microparticles represent cell membrane obtained particles that are comprised of vasoconstrictive agents like prostaglandin E2, thromboxane A2, inflammatory modulators, procoagulants as well as fibrin. Hence microparticles might work in the form of localized generators of Oxidative stress, inflammation along with disseminated intravascular coagulation that causes vascular congestion [35]. It can be considered that these microcirculatory disconcertion might get exaggerated in particular, amongst the smaller arteries of the renal medullary vasa recta that might reason out why the renal medulla is prone with regards to the generation of hypoxia at the time of sepsis.
Hypoxia of the renal medullary tissue: a key process in acute Kidney injury?
Hypoxia of the renal medullary tissue is being probed in the form of a usual pathophysiological characteristic of AKI that arises from sepsis [10,36], cardiopulmonary bypass [37], along with radiocontrast induced Nephropathy [38]. Moreover, hypoxia of the renal medullary tissue has been believed to be a significant stimulator with regards to transition, as well as, chances for propagation from AKI to CKD [39]. The proportionally high metabolic needs of the tubular elements that are existent in the renal medulla, that are coupled with the terrain of vascular along with tubular engineering amongst the medulla cause a steep oxygen gradient amongst the capillaries (vasa recta) in addition to both the thick as well as thin ascending loop of Henle along with the collecting ducts [40]. Furthermore, the probability for diffusive oxygen shunting amongst the renal medullary microcirculation (from descending to ascending vasa recta) that might further negotiate the renal medullary oxygen supply [41]. Graded clamping of the renal arteries in case of healthy sheep, thus result in a continuous drop in renal blood flow caused a greater amount of renal medullary ischemia, as well as, hypoxia as per percentage in contrast to the renal cortex that pointed to an internal deficiency with regards to the autocontrolling ability of the renal medullary microcirculation [42]. Thus at the time of pathophysiological situations like sepsis, renal microcirculation disturbances that result in just modest decreases in medullary oxygen supply or escalation in renal oxygen utilization might have bad sequelae for the medullary tissue oxygenation.
Renal medullary hypoxia might be a robust stimulator of stepwise processes that result in cellular damage, vascular damage along with, tubular impairment [43]. Any acute insults that is inclusive of endotoxemia possess the capacity of escalation of renal tissue oxygen utilization. Like these alterations possess the capacity of causing tubular damage besides compression, along with inappropriate localization of Na/K ATPase as well as transportation proteins amongst renal tubular epithelial cells thus result in a reduction of the effectiveness of the oxygen consumption with regards to sodium reabsorption [44]. Furthermore, it is of significance that Oxidative stress can cause escalation of utilization of tubular oxygen by a reduction in effectiveness of the ATP generation amongst the mitochondria, as well as/or by escalation of oxygen consumption with regards to tubular sodium reabsorption [45]. Furthermore, renal insults that acutely arbitrate the vascular intactness or continuously facilitate vascular compressive/blockade action alias rarefaction might result in reduction of renal medullary oxygen supply that might result in deterioration of the tissue hypoxia. In a clinically appropriate ovine model of gram-negative sepsis the generation of renal medullary hypoxia anteceded AKI, as estimated by the escalation of plasma creatinine along with oliguria by 12-24 h [10,11]. Bladder urinary oxygenation got corroborated in the exact model that was utilized earlier. For the provision of a dependable evaluation of renal medullary tissue oxygenation at the time of generation of septic AKI [46] along with in reaction to clinical management, that is inclusive of building up the patients, with fluids [47], vasopressin [11], as well as, diuretics [48]. Subsequently, in case of human studies, the existence of renal tissue medullary hypoxia, as indirectly evaluated by estimation of bladder urinary hypoxia was illustrated in patients with septic AKI [49]. Cellular modes that confer protection, stimulated by HIFs possess the capacity of protection of the Kidneys once there is mild tissue hypoxia/or temporary, however, they might crash if tissue hypoxia becomes robust/or continued as might take place at the time of stress [50].
HIFs represent cellular oxygen detectors. Stabilization of the α subunit at low oxygen amounts causes the generation of a dimer with the β subunit. Subsequently, the dimer gets translocated towards the nucleus, binding to the hypoxia response element that causes changed transcription of numerous proteins that are necessary for numerous crucial signaling pathways [51]. This upregulation in addition to stabilization of the HIFs is considered to be adaptive, as it causes stimulation along with a generation of erythropoietin that facilitates the generation of red blood cells [52]. The escalation of the blood oxygen carrying ability thus, it possesses the capacity of abrogation of tissue hypoxia. ii) The other significant protein which gets upregulated secondary to the stabilization of the HIFs is eNOS that has the probability of causing an escalation of the NO bioavailability, thus mitigating vasoconstriction, which restricts greater progression of hypoxia along with ROS/RNS [51]. Nevertheless, HIFs might work in the form of a double-edged weapon in view of accelerated generation of HIFs in reaction to continued hypoxia at the time of robust sepsis might cause exhaustive generation of vasoconstrictive in addition to ROS stimulated proteins, like Inos [53], besides proteins that result in fibrogenesis [54]. Relevant to these findings hypoxia at the time of early initiation of renal tissue medullary hypoxia during sepsis causes prolongation of phases of tissue hypoxia which can result in destabilization of the HIFs, which result in prolongation of Oxidative as well as nitrosative damage, that ends in AKI. Validating this belief HIF-1α gene got down regulated amongst the renal medulla in case of septic sheep with robust AKI subsequent to 48 h of sepsis [8].
It is the knowledge we possess that the mode amongst renal medullary hypoxia along with a reduction in glomerular filtration rate (GFR) has not got found in AKI as well as might vary based on the etiology of AKI. Nevertheless, it might be thought that continued tissue hypoxia specifically in case of metabolically active areas of the Kidney, like renal medulla, result in mitochondrial impairment, that causes escalation of ROS generation which further results in augmentation of renal cellular damage.
Mitochondrial impairment secondary to sepsis stimu-lates generation of ROS
It has been posited that mitochondrial impairment is the etiology apart from the pathogenesis of septic AKI [55]. Mitochondria remain the ones that cause the utilization of oxygen maximum in the kidney. Hence, the generation of physiological amounts of mitochondrial ROS in the mitochondrial matrix is significant as ROS work as signals in addition to controllers of numerous biological events. These are inclusive of getting adapted to hypoxia via the control of the stabilization of the HIFs [56], ii) promote the generation of autophagosomes via the Oxidation of the cysteine protease gene4 by H2O2 [57], along with iii) ROS based stimulation of phosphatidyl inositide 3-kinase (PI3K) that results in the downstream generation of proinflammatory cytokines that are inclusive of caspase 1, IL-1β, as well as, IL-18 [58]. Nevertheless, with continuous exposure of the cells to hypoxia, alterations in metabolism, apart from bad oxygen consumption with regards to ATP generation in the mitochondrial electron transport chain (ETC), that causes an escalation of leakage of electrons along with escalation of generation of free radicals/ROS [59]. Oxygen consumption by mitochondria occurs in the form of the ultimate acceptor of the respiratory chain, however, its incomplete reduction can further generate ROS, in particular superoxide [60]. Complex IIIof the electron transport chain (ETC), represent an inherent oxygen detector at the time of acute hypoxia, besides its control of superoxide generation inversely as per oxygen being available [61]. The conversion of Complex I from its active towards inactive form was further documented for possessing the capacity of generation of ROS outbursts at the time of acute hypoxia [81]. Septic AKI patients possess escalation of receptor interacting protein kinase (RIPK3) in urine along with plasma [62]. RIPK3 facilitates Oxidative stress in addition to mitochondrial impairment in Kidney tubular epithelial cells by an escalation of the expression along with mitochondrial transportation of NADPH oxidase as well as hampering of mitochondrial complexes I as well as III [63]. Thus it is not astonishing that mitochondrial damage has been usually associated with multiorgan impairment in case of patients with sepsis [64].
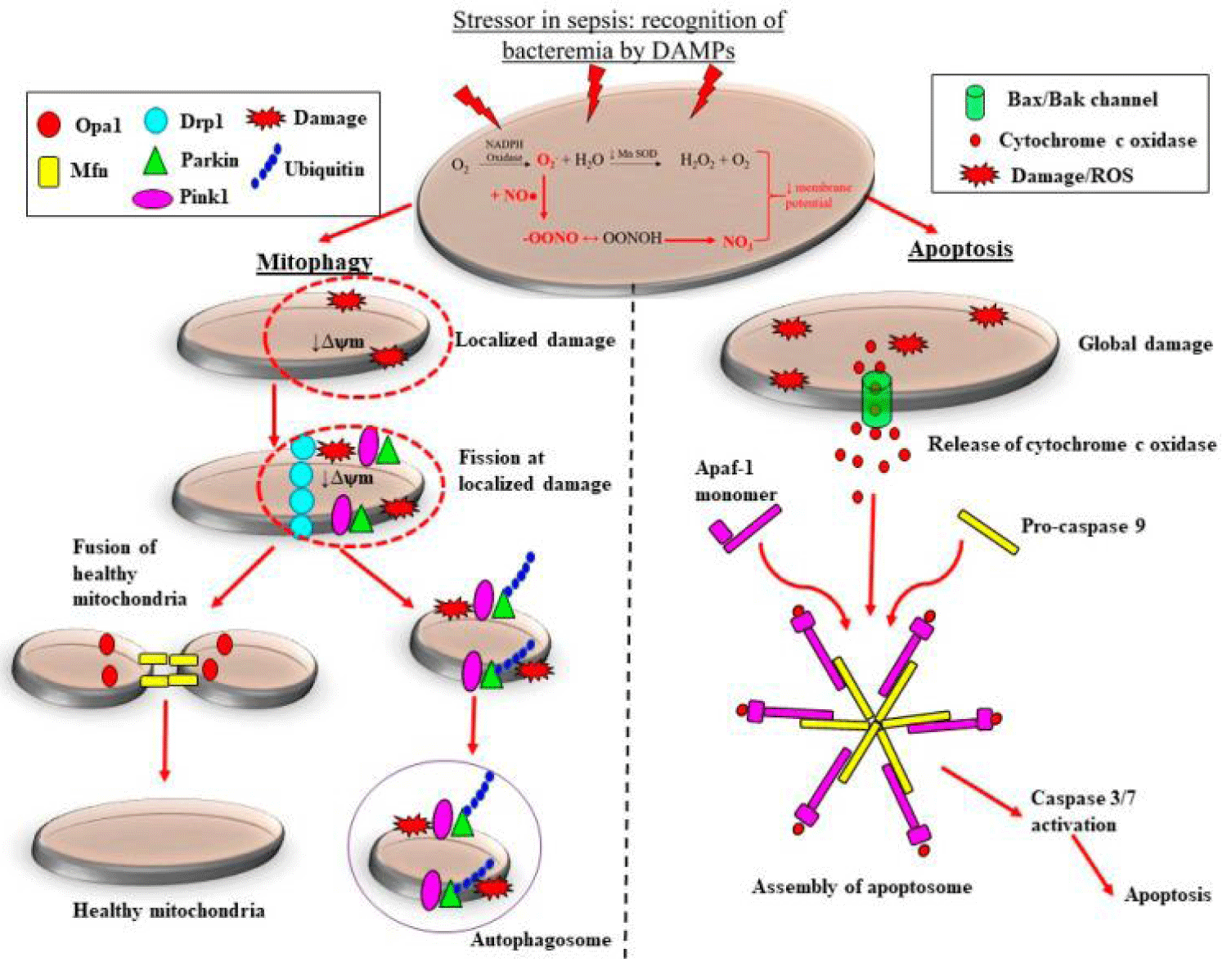
Preclinical along with clinical studies illustrate that the adaptive events of mitochondrial fission get downregulated in sepsis, that probably aids in the deletion of mitochondrial mass, thus aid in progression of ROS stimulated damage at the time of septic AKI. Sepsis gets correlated with marked morphological alterations in mitochondria. These alterations are inclusive of reduction in numbers of cristae that is secondary to the swelling of intercristal space in addition to the mitochondrial matrix along with vacuolation amongst the mitochondrial space [63,65]. On the basis of the robustness of mitochondrial injury, the depletion of mitochondria might be brought about by 2 pathways namely mitophagy, as well as, apoptosis (Figure 3). Localized in addition to exhaustive morphological abnormalities amongst the mitochondria can result in the collection of superoxide ultimately resulting in the opening of the mitochondrial membrane channels. This results in reduction of the mitochondrial membrane potential, that is restricted towards the area of damage, thus further result in escalation of generation of ROS [66]. Moreover, escalation of amounts along with/or collection of up regulation of the uncoupling protein-1 [67], which as a consequence cause an escalation of proton leakage, as well as, inhibiting the generation of ATP [68]. Here in view of the injury having a tendency of being localized, the mitochondrion getting targeted for mitochondrial fission that is subsequently followed by the deletion of the injured part of the mitochondrion by mitophagy (Figure 3). At the time of mitochondrial fission, the mitochondrial membrane gets pinched at the damaged region that gets promoted by the assembling of dynamin associated protein1 which promotes the deletion of the injured area of the mitochondria from the noninjured area (Figure 3) [69]. Subsequently PTEN stimulated kinase-1 along with ubiquitin-protein ligase (Parkin) proteins recruitment takes place towards the injured mitochondria that finally result in generation of the autophagosomes, thereby getting depleted by mitophagy [69]. Mitochondrial fusion proteins mitofusin in addition to mitochondrial dynamin like GTPase, get recruitment towards the left healthy mitochondrion (Figure 3). Hence, mitochondrial fusion that is followed by fission, is apparently a significant adaptive healing event, as besides putting a halt to subsequent mitochondrial injury in addition to avoidance of escalation of depletion of the mitochondrial mass, thus restricting ROS stimulated damage. Validating this belief, reduction in expression of mitochondrial PINK1 along with Parkin mRNA has been documented in biopsies from patients having mortality secondary to septic AKI [70]. Exhaustive injury to the mitochondria causes global fall of mitochondrial permeability that might result in superoxide collection, that finally result in opening of the mitochondrial membrane channels. These damages stimulate a cascade of downstream processes that is initiated by the liberation of pro-apoptotic factors like Bcl2 correlated X protein BAX as well as B cell lymphoma protein. This causes modulation of the liberation of cytochrome c, that in turn crosstalks with the apoptotic proteaseactivating factor1 proteins, that finally causes generation of an apoptosome which stimulate a procaspase 9-modulated downstream inherent apoptotic cascade [71] (Figure 3). This event promotes the depletion of the whole mitochondria in robust sepsis, as well as, causes decrease in mitochondrial mass [70] that might further arbitrate the generation of host antioxidants along with escalate the generation of superoxide (Figure 3). Hence ROS stimulated damage of the mitochondria in sepsis can cause escalation of ROS generation as well as collection that might aid in a vicious progressing cycle of Oxidative stress, macrovascular damage along with cellular damage that ends in AKI. Thus atleast in theoretical manner the generation of Pharmacologic treatment with the objective of halting as well as /or restriction of generation of along with the collection of ROS/ as well as, or RNS at the time of sepsis might ameliorate the progression of vascular, mitochondrial, as well as, cellular damages, as well as, restrict the robustness of septic AKI. Here details of preclinical along with clinical studies which have evaluated the utilization of NAC or Vitamin C in the form of a therapeutic approach for mitigation of Oxidative stress stimulated multiorgan impairment in sepsis are discussed.
Figure 3: Courtesy ref no-15-Adaptive and maladaptive reactions of the mitochondria to sepsis- stimulated oxidative stress. Escalated generation of superoxide (O2-) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase coupled with the reduction in action of manganese superoxide dismutase (MnSOD) causes accrual of O2-. Cytosolic nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) reacts with O2-, forming the highly reactive peroxynitrite (ONOO−). The accrual of O2- and ONOO− causing continued oxidative stress along with adecrease in mitochondrial membrane potential (ψm) and so mitochondrial impairment. In the case of localized mitochondrial injury, mitochondrial quality control modes are activated which arrest mitochondrial impairment. In addition to restrict escalated mitochondrial depletion. Recruitment of mitochondrial fission proteins to sites of damage targets the injured regions of the mitochondrion for fission. Subsequently, ubiquitin, PTEN-induced kinase (PINK1) and E3 ubiquitin-protein ligase (Parkin) proteins areenrolled to the injured mitochondrion, removed by mitochondrial fission and crosstalk with phagophore, consequently generating an autophagosome. The healthy regions of the mitochondrion undergoes mitochondrial fusion, that adds to the existing mitochondrial pool and restricting escalated mitochondrial depletion. Conversely, Comprehensive injury to mitochondria in robust, sepsis causes the liberation of cytochrome c oxidase, along with in the f generation of an apoptosome when it crosstalks with apoptotic protease activating factor-1 (Apaf-1) monomers as well as pro-caspase 9. This results in downstream activation of caspase 3/7, ultimately causing the containment of sepsis-induced injury via apoptosis. Mitochondrial dynamin-like GTPase (OPA-1); dynamin related protein-1 (DRP-1); damage-associated molecular patterns (DAMPs).
N-acetyl cysteine
NAC represents a robust synthetic antioxidant that forages superoxide, H2O2, besides hydroxyl radicals in addition to the restoration of glutathione amounts, thus escalating the antioxidant reactions of the host [72]. NAC has been demonstrated to cause repression of production of oxygen radicals by the human neutrophils as well as macrophages in vitro [73], cause reduction in leukocyte to endothelial cells adhesion, as well as, vascular leakage in case of rodent endotoxemia [74] besides ameliorate the activation of nuclear factor κB (NFκB) along with IL-8 amounts in sepsis [75]. These observations yielded the stimulus for evaluation of NAC for reversal of the harmful actions of Oxidative, as well as, nitrosative stress in sepsis.
Evaluation of NAC has numerous experimental studies in the form of precautionary treatment or rescue treatment at the time of early stages of sepsis. In case of a rodent model of sepsis that has been stimulated by cecal ligation as well as puncture (CLP), subcutaneous NAC in a dosage of 20 mg/kg therapy started 3 h subsequent to CLP caused a reduction in systemic inflammation, Oxidative stress along with mitochondrial impairment that results in a significant escalation of survival (10% - 40%) [76]. In case of rodent endotoxemia intravenous NAC treatment) started 20’ subsequent to delivery of lipopolysaccharides (LPS) caused a reduction in serum creatinine along with blood urea nitrogen amounts, an action correlated with a decrease in inflammatory cytokines (TNFα as well as IL-6) [77]. Nevertheless, treatment started at a markedly early stage of sepsis are of restricted value with regards to a clinical scenario. This is in view of patients with sepsis get usually diagnosis made with, the recipient of management, once there is the existence of a proven multiorgan impairment, as estimated by sequential organ failure assessment (SOFA) scores [78]. In this context intravenous treatment with NAC (150 mg/kg bolus+20 mg/kg/h) in pigs 12 hrs subsequent to development of endotoxemia could not respond with no enhancement in systemic, pulmonary, as well as, hepatospanchnic hemodynamics or be correlated with the reduction in a biomarker of Oxidative stress (8-isoprostane), inspite of escalation of glutathione amounts [79].
Clinical studies with the utilization of NAC as ancillary treatment for stress have mostly resulted in poor results specifically when NAC was delivered to patients with robust sepsis for longer periods. In maximum patients, the utilization of NAC was done in the dosage of (150 mg/kg) that was dependent on the regimen that was used in patients who got treatment for acetaminophen poisoning [80]. In case of patients with sepsis, intravenous NAC delivered over a short duration relatively (150 mg/kg bolus + 12.5 mg/kg/h for 90’) resulted in significant escalation of cardiac index (5.7 (treatment) vs. 5.0 (usual care) L/min/m2, p = 0.01). Nevertheless, NAC delivery for longer periods of time (50 mg/kg at 4 h that was followed by 100 mg/kg/24 h for 44 h) resulted in deterioration of organ failure (average SOFA scores at 48 h: 7.7 ± 3.8 (treatment) vs. 5.1 ± 2.1 (usual care) L/min/m2, p < 0.05) without any enhancement of microalbuminuria/ creatinine ratio in patients with sepsis [81]. As far as NAC delivered to patients with the diagnosis of septic shock made for time periods greater than 24 h resulted in cardiac depression that had the properties of reduction in cardiac output along with enhancement of hypotension [82]. Akin to that greater needs with regards to inotropic support got illustrated in critically ill patients who received treatment with NAC (150 mg/kg bolus + 12 mg/kg/h) for periods over 24 h in contrast to that with usual care [83]. The maximum dosage of NAC delivered to patients with the diagnosis of sepsis (3 g 6 hrly x 3 days) did not result in significant enhancement with regards to patient mortality, however, possessed harmful actions that was inclusive of escalation of the risk of the inflammation that was correlated with deteriorated AKI [84]. Nevertheless, at present there is a deficiency of pharmacokinetics along with pharmacodynamics results with regards to NAC as far as initiation, disease propagation, as well as, the robustness of sepsis is not clear. Hence further clinical in addition to experimental studies are needed prior to abandonment of the advantageous actions of NAC is considered (Table 1).
| Table 1: On actions of N-Acetyl Cysteine (NAC)in case of oxidative stress pertaining to sepsis in both models. | ||||||
| Reference no | Author name | Effects of NAC |
Mode of actions (Antioxidants influenced |
Radicals influenced |
Restoration of physiological substances | Efficacy |
| 72. | Arouma, et al. 1989 | NAC(synthetic) | Robust Antioxidants |
superoxide, H2O2. hypochlorous acid, | glutathione amounts | |
| 73. | Kharazmi, et al. 1988 | ditto | cause repression of production of oxygen radicals by the human neutrophils as well as macrophages in vitro hampering of neutrophils as well as chemotaxis | |||
| 74. | Schmidt, et al. 1997 | cause reduction in leukocyte to endothelial cells adhesion as well as vascular leakage in case of rodent endotoxinemia | ||||
| 75. | Patterson, et al. 2003 | ameliorate the activation of nuclear factor κB (NFκB) along with IL-8 amounts in sepsis | observations yielded the stimulus for evaluation of NAC for reversal of the harmful actionsof observations yielded the stimulus for evaluation of NAC for reversal of the harmful actions of Oxidative as well as nitrosative stress in sepsis . | |||
| 76. | Ritter, et al. 2004 | NAC + desferoxamine | a rodent model of sepsis that has been stimulated by cecal ligation as well as puncture (CLP) | subcutaneous NAC in a dosage of 20 mg/kg therapy started 3 h subsequent to CLP caused a reduction in systemic inflammation, Oxidative stress along with mitochondrial impairment that result in significant escalation of survival (10% - 40%) | ||
| 77. | HsuBG et al.2006 | rodent endotoxinemia | intravenous NAC treatment) started 20’ subsequent to delivery of lipopolysaccharides (LPS) caused reduction in serum creatinine along with blood urea nitrogen amounts, an action correlated with decrease in inflammatory cytokines (TNFα as well as IL-6). | |||
| 78. | Singer, et al. 2016 | Nevertheless, treatment started at markedly early stage of sepsis are of restricted value with regards to a clinical scenario. This is in view of patients with sepsis get usually diagnosis made with, recipient of management, once there is existence of proven multiorgan impairment, as estimated by sequential organ failure assessment (SOFA) scores | ||||
| 79. | Vassilev, et al. 2004 | context intravenous treatment with NAC (150 mg/kg bolus+20 mg/kg/h) in pigs 12 hrs subsequent to development of endotoxinemia could not respond with no enhancement in systemic, pulmonary, as well as hepatospanchnic haemodynamics or be correlated with reduction in a biomarker of Oxidative stress (8-isoprostane), inspite of escalation of glutathione amounts | ||||
| 80. | Szakmany, et al. 2007 |
Clinical studies with utilization of NAC as anciliary treatment for stress have mostly resulted in poor results specifically when NAC was delivered to patients with robust sepsis for longer periods. In maximum patients the utilization of NAC was done in the dosage of (150 mg/kg ) that was dependent on the regimen that was used in patients who got treatment for acetaminophen poisoning | ||||
| 81. | Spapen, et al. 2005 | In case of patients with sepsis, intravenous NAC delivered over a short duration relatively (150 mg/kg bolus+12.5 mg/kg/h for 90’) resulted in significant escalation of cardiac index (5.7 (treatment) vs. 5.0(usual care) L/min/m2, p = 0.01). Nevertheless, NAC delivery for longer periods of time (50 mg/kg at 4 h that was followed by 100 mg/kg/24 h for 44 h) resulted in deterioration of organ failure(average SOFA scores at 48 h: 7.7 ± 3.8 (treatment) vs. 5.1 ± 2.1 (usual care) L/min/m2, p < 0.05) without any enhancement of microalbuminuria/creatinine ratio in patients with sepsis |
||||
| 82. | Peake, et al.1997 | NAC delivered to patients with the diagnosis of septic shock made for time periods greater than 24 h resulted in cardiac depression, that had the properties of reduction in cardiac output along with enhancement of hypotension | ||||
| 83. | Molnar, et al.1999 | Akin to that greater needs with regards to inotropic support got illustrated in critically ill patients who received treatment with NAC (150 mg/kg bolus+12 mg/kg/h) for periods over 24 h in contrast to that with usual care | ||||
| 84. | Najafi. et al. 2014 | The maximum dosage of NAC delivered to patients with the diagnosis of sepsis (3 g 6 hrly x 3 days) did not result in significant enhancement with regards to patient mortality, however, possessed harmful actions that was inclusive of escalation of the risk of inflammation that was correlated with deteriorated AKI,]. Nevertheless, at present there is deficiency of pharmacokinetics along with pharmacodynamics results with regards to NAC as far as initiation, disease propagation as well as robustness of sepsis is not clear. Hence further clinical in addition to experimental studies are needed prior to abandonment of the advantageous actions of NAC is considered. | ||||
Vitamin C
Vitamin C represents a necessary antioxidant, that hunts ROS in addition to RNS [85], besides resulting in restoration of the endogenous glutathione amounts, thus causing restoration of host’s antioxidant defenses [86]. Akin to NAC, Vitamin C possesses antiinflammatory actions by hampering the actions of nuclear factor κB (NFκB) along with generation of proinflammatory modulators [87]. Nevertheless, not like NAC, there is an escalation of proof that Vitamin C, that possesses actions other than its effect as a Vitamin, in the form of a stress hormone, might be playing a key part in the modulation of the adrenocortical stress response, specifically in sepsis, that is it possesses pleiotropic characteristic s. Vitamin C, acts in the form of an immune stimulator by which it causes enhancement of macrophages [88], besides might possess the capacity of direct hampering of bacterial proliferation [89]. In cultured human endothelial cells, Vitamin C in a dose-based manner caused hampering of the TNFα stimulated expression of intracellular adhesion molecule [ICAM]-1, thus it possesses the capacity of reduction of microvascular leukocyte that plug in addition to microcirculatory impairment [90]. Vitamin C further has the capacity of reversing BH4oxidation along with the escalation of BH4 based generation of nitric oxide(NO) from eNOS [91], as well as/or escalate eNOS bioavailability through fast phosphorylation of eNOS-Ser1177 [92], actions not observed with antioxidants like NAC [93]. Furthermore, Vitamin C, acts as a significant cofactor for the generation of endogenous vasoconstrictors, like noradrenaline, as well as, vasopressin [94], that might further help. In the circulatory treatment of patients with sepsis, that commonly develop nonresponsiveness towards vasopressor treatment. Critically ill patients possess aberrantly low amounts of ascorbate [95] that gets further worsened in humans not possessing the capacity of generation of Vitamin C, that gives one extra reason for Vitamin C supplementation, in patients with sepsis [96]. In view of enteral uptake being not enough for normalization of amounts of ascorbate in view of saturable intestinal Sodium dependent Vitamin C cotransporters (SVCTs), the need for intravenous therapy exists [97].
Experimental studies have given sufficient proof with regards to the possible advantages of intravenous Vitamin C in sepsis. Murine sepsis that gets stimulated by CLP, pretreatment with Sodium ascorbate(200 mg/kg), avoided vascular leaking by hampering the induction of iNOS superoxide generation along with peroxynitrite generation in skeletal muscle tissue [98]. Sodium ascorbate resulted in a reduction of CLP stimulated superoxide development by avoidance of NADPH oxidase activation in addition to uncoupling eNOS in the same study [98]. Ascorbate treatment in mice with fecal induction of peritonitis (FIP), 6 h post FIP resulted in reversing of platelets adhesion, enhancement of muscle capillary blood distribution along with the escalation of survival, however, these advantages were not visualized in eNOS knockout mice [99]. Pre-treatment with sodium ascorbate (76 - 200 mg/kg), has been documented to ameliorate the reduction in pressor reactions to vasopressors that is inclusive of noradrenaline, as well as, angiotensin II that have been visualized in CLP mice through hampering of iNOS action [100]. These experimental findings have yielded the scientific basis for clinical trials that evaluated the provisional advantages of Vitamin C in human sepsis.
Inspite of the posited advantages of Vitamin C that have been evaluated in animal models of early stage sepsis, its effectiveness in human sepsis is contradictory. 2 small, single-center randomized clinical trials (RCT; n = 24 & n = 28), demonstrated, that intravenous infusion of ascorbic acid, at dosages varying from 50 - 200 mg/kg/day, caused a reduction in inflammatory markers, as well as, sequential organ failure assessment (SOFA) scores [101] along with enhancement of vasopressor sensitivity (25 mg/kg 6 hrly x 3 days) [102]. One more single centre retrospective prior, as well as, subsequent study (n - 47) with the utilization of a combination treatment of ascorbic acid (1.5 g 6 hrly) along with hydrocortisone as well as thiamine isolated significant drop with regards to hospitalized mortality (8.5% vs. 40,4%, p < 0.001), time period of vasopressor utilization (18.3 ± 9.8h, s, 54.9 ± 28.4 h%, p < 0.001), with the need for RRT (10% vs. 37,4%, p < 0.05) [103], Nevertheless, further multicentre RCT (10% vs. 37, 4%, p < 0.05) at 72 h following the; [103]. Nevertheless, further multicentre RCT, VITAMINS [104], ACTS [105] as well as ATESS [106] in which trials of ascorbic acid 6 g daily with thiamine, with or without corticosteroid for upto 10 days, did not possess the capacity of estimation the variation in mortality or need for RRT in cases of sepsis. The CITRIS ALI trial where utilization of 16 g daily of intravenous ascorbic acid for 4 days in cases of sepsis, further were unable to find enhancement of average modified SOFA score, despite a decrease in 28 days mortality from 46% - 30% was seen [107]. Sepsis possesses the properties of a significant systemic inflammation status that has been demonstrated to cause downregulation of cellular SVCTs [108]. As per this, greater dosages in contrast to those previously trialed in clinical sepsis might be needed to tide over the reduction in SVCTs, besides pushing Vitamin C into cells for attaining its intracellular advantages.
As significantly greater dosages of Vitamin C have been documented to possess safety in cancer patients or those with burns [109], the preclinical safety along with the effectiveness of a mega dosage of Sodium ascorbate (150 g for a 40 kg sheep) was more recently, evaluated in an ovine model of sepsis with proved AKI [110]. Subsequent to 24 h of proved sepsis, mega dosage of Sodium ascorbate therapy reversed renal medullary ischemia or hypoxia in septic sheep that was associated with a total reversion of AKI, as estimated by marked escalation of urine flow, as well as, creatinine clearance besides the return of plasma creatinine to normal values [110]. Furthermore, Sodium ascorbate significantly resulted in a reduction in the needs for noradrenaline required, for the restoration of target blood pressure (BP), cause a reduction of arterial blood lactate amount besides enhancement of lung function in septic sheep [137]. Additionally, a critically ill septic patient with COVID-19 stimulated hypotension, as well as, AKI got treated with intravenous Sodium ascorbate (60 g) used on compassionate grounds. Akin to septic sheep Sodium ascorbate resulted in a reduction in plasma creatinine (118 to 84 μmol/l) besides escalation of urine flow (10 – 400 ml/h, besides average arterial pressure restoration inspite of total removal of noradrenaline, for a minimum period of 7 h intervention duration [110]. In total these preclinical as well as, clinical studies pointed that the ideal dosage, timing in addition to time period of Vitamin C treatment are probably the key factors which estimate its treatment effectiveness, in case of sepsis, however, these factor at present require further evaluation, in details [96]. Thus, 2-pilot placebo controlled RCT are being evaluated at present for the actions of intravenous mega dosage of Sodium ascorbate (60 g as well as 120 g) treatment (on renal outcomes besides vasopressor needs in patients with sepsis (ACTRN1262000651987p; NCT04796636) (Table 2).
| Table 2: On actions of Vitamin C on Oxidative stress in Sepsis (in human &animal models. | ||||||
| Reference no | Author | Effects of Vitamin C |
Mode of action | Radicals foraged | Restoration of physiological substances |
|
| 85. | Padayatty, 2016 | antioxidant | hunts ROS & RNS | restoration of the endogenous glutathione amounts | Thus antioxidant Status of host |
|
| 86. | Buetner, et al. 1996 | Besides antioxidant |
It can act as a prooxidant as a reducing agent to reduce redox-activemetal. s such as copper and iron, | |||
| 87. | Carcamo, et al. 2002 | Akin to NAC, Vitamin C possesses antiinflammatory actions by hampering the actions of nuclear factor κB(NFκB) along with generation of proinflammatory modulators | Vitamin Causes repression of TNF-α stimulated NFκB activation, by hampering I kappa B phosphorylation | |||
| 88. | Victor, et al. 2000 | peritoneal macrophages from BALB/c mice suffering lethal endotoxic shock caused by intraperitoneal (i.p.) injection of Escherichia coli lipopolysaccharide (LPS; 100 mg/kg) a high production of superoxide anion | The increased adherence, ingestion and superoxide anion production by macrophages from animals with endotoxic shock were lower in the presence of AA, reaching similar values to those of the control animals. | Vitamin C in various doses showed 0.01 nm most efficacious | ||
| 89. | Armour, et al. 2001 | Nevertheless, not like NAC, there is escalation of proof that Vitamin C, that possesses actions other than its effect as a Vitamin, in the form of a stress hormone, might be playing key part in the modulation of adrenocortical stress response, specifically in sepsis, that is it possesses pleiotropic characteristics. Vitamin C, acts in the form of an immune stimulator by which it causes enhancement of | In case of CLP animals iv ascorbate restored Vitamin C, amounts hampered replication of bacteria &avoided H2O2 induced cultured microvascular endothelial cells injury | Thus vit c lost in urine & iv vit c can restore skeletal. muscle function | ||
| 90. | Mo, et al. 2003 | In cultured human endothelial cells, Vitamin C in a dose based manner caused hampering of the TNFα stimulated expression of intracellular cell endothelial cells adhesion molecule [ICAM]-1, thus it possess the capacity of reduction of microvascular leukocyte that plug in addition to microcirculatory impairment | ||||
| 91. | Kim, et al. 2006 | In cultured human endothelial cells, Vitamin C in a dose based manner caused hampering of the TNFα stimulated expression of intracellular cell endothelial cells adhesion molecule [ICAM]-1, thus it possess the capacity of reduction of microvascular leukocyte that plug in addition to microcirculatory impairment | ||||
| 92. | Ladurner, et al. 2003 | as /or escalate eNOS bioavailability through a fast phosphorylation of eNOS-Ser1177i i. | ||||
| 93. | Schneider, et al. 2005 | actions not observed with antioxidants like NAC | ||||
| 94. | Carr, et al. 2005 | Acts as a cofactor for the generation of endogenous vasoconstrictors, like noradrenaline as well as vasopressin | Thus helpful in sepsis & septic shock for sustenance of BP. | |||
| 95. | Carr, et al. 2018 | that might further help In the circulatory treatment of patients with sepsis,that commonly develop nonresponsiveness towards vasopressor treatment. Critically ill patients possess aberrantly low amounts of ascorbate [Hypo Vitaminosis C generation & deficiency | Despite advocated togienteral & parenteral intakes in these critically ill patients | |||
| 96. | May, et al. 2021 | Megadose of Vitamin C | Conscious sheep received an infusion of live Escherichia coli for 31 hours. At 23.5 hours of sepsis, | in all animals, sodium ascorbate (0.5 gm/kg over 0.5 hr or 0.5 g/kg/hr x 6.5 hrs dramatically improved the |
Fever, HR, creatinine all reducdinc in renal medullary pO2.decin noradr vasopressordose | |
| 97. | Wilson, et al. 2013 | In view of enteral uptake being not enough for normalization of amounts of ascorbate in view of saturable intestinal Sodium dependent Vitamin C cotransporters (SVCTs),with need for intravenous therapy | ||||
| 98. | Zhou, et al. 2012 | Murine sepsis that gets stimulated by CLP (cecal ligation and puncture | Pretreatment with Sodium ascorbate (200 mg/kg), avoided vascular leaking by hampering the induction hampering of iNOS superoxide generation along with peroxynitrite generation in skeletal. muscle tissue | Sodium ascorbate resulted in reduction of CLP stimulated superoxide development by avoidance of NADPH oxidase activation in addition to uncoupling eNOS in the same study | ||
| 99. | Tym, et al. 2008 | Ascorbate treatment in mice with faecal induction of peritonitis (FIP), 6 h post FIP resulted in reversing of platelets adhesion, enhancement of muscle capillary blood distribution along with escalation of survival, however these advantages were not visualized in eNOS knockout mice |
||||
| 100. | Wu, et al. 2004 | Pre treatment with sodium ascorbate (76-200 mg/kg), has been documented to ameliorate the reduction in pressor reactions to vasopressors that is inclusive of noradrenaline as well as angiotensin II that have been visualized in CLP mice through hampering of iNOS action. | These experimental findings have yielded the scientific basis for clinical trials that evaluated the provisional advantages of Vitamin C in human sepsis. | |||
| 101. | Fowler, et al. 2014 | Inspite of the posited advantages of Vitamin C that have been evaluated in animal models of early stage sepsis, its effectiveness in human sepsis is contradictory. 2 small, single center randomized clinical trial (RCT; n = 24 & n = 28), demonstrated, that intravenous infusion of ascorbic acid ,at dosages varying from 50-200 mg/kg/day ,caused reduction in inflammatory markers as well as sequential organ failure assessment (SOFA) scores |
||||
| 102. | Khalili, et al. 2016 | along with enhancement of vasopressor sensitivity (25 mg/kg 6 hrly x 3 days) | ||||
| 103. | Marik, et al. 2017 | One more single centre retrospective prior as well as subsequent study (n - 47) with the utilization of a combination treatment of ascorbic acid (1.5 g 6 hrly) along with hydrocortisone as well as thiamine isolated significant drop with regards to hospitalized mortality (8.5% vs. 40,4%, p < 0.001), time period of vasopressor utilization (18.3 ± 9.8h,s, 54.9 ± 28.4h%, p < 0.001), with the need for RRT (10% vs. 37,4%, p < 0.05)[ |
Nevertheless, further multcentre RCT (10% vs. 37,4%, p < 0.05) at 72 h following the Intevention did not corraborate |
|||
| 104. | Fuji, et al. 2020 | further mult centre RCT, VITAMINS | In which trials of ascorbic acid 6 g daily with thiamine, with or without corticosteroid for upto 10 days | Did not possess the capacity of estimation the variation in mortality or need for RRT in cases of sepsis | ||
| 105. | Moscowitz, et al. 2020 | Effects of ascorbic acid, corticosteroids and thiamine on organ injury in septic shock (ACTS RCT) | ,, | |||
| 106. | Hwang, et al. 2020 | Combination treatment of Vitamin C and thiamine for septic shock; a multicentre, double blinded randomized controlled study ATESS RCT |
DITTO | |||
| 107. | Fowler, et al. 2019 | CITRIS ALI trial where utilization of 16 g daily of intravenous ascorbic acid for 4 days in cases of sepsis, further were unable to find enhancement of average modified SOFA score, despite a decrease in 28 days mortality from 46% - 30% was seen |
||||
| 108 | Subramanium, et al. 2019 | Sepsis possesses the properties of a significant systemic inflammation status, that has been demonstrated to cause downregulation of cellular SVCTs | TNF-α reduces intestinal Vitamin C absorption |
|||
| 109 | Yahase, et al. 2021 | As significantly greater dosages of Vitamin C have been documented to possess safety in cancer patients or those with burns | ||||
| 110 | Lankadeva, et al. 2021 | the pre-clinical safety along with effectiveness of a mega dosage of Sodium ascorbate (150 g for a 40 kg sheep)was more recently, evaluated in an ovine model of sepsis with proved AKI | Subsequent to 24 h of proved sepsis, mega dosage of Sodium ascorbate therapy reversed renal medullary ischemia or hypoxia in septic sheep, that was associated with a total reversion of AKI [as estimated by marked escalation of urine flow as well as creatinine clearance besides return of plasma creatinine to normal values[ |
Furthermore, Sodium ascorbate significantly resulted in reduction in the needs for noradrenaline required, for the restoration of target blood pressure (BP), cause reduction of arterial blood lactate amount besides enhancement of lung function in septic sheep | Additionally, a critically ill septic patient with COVID-19 stimulated hypotension as well as AKI got treated with intravenous Sodium ascorbate (60 g) used on compassionate grounds. Akin to septic sheep Sodium ascorbate resulted in reduction in plasma creatinine (118 to 84 μmol/l) besides escalation of urine flow (10-400 ml/h, besides average arterial pressure restoration inspite of total removal of noradrenaline, for a minimum period of 7 h intervention duration | |
| Summing up | In total these preclinical as well as clinical studies pointed that the ideal dosage, timing in addition to time period of Vitamin C treatment are probably the key factors which estimate its treatment effectiveness, in case of sepsis, however these factor at present require further evaluation, in details | Thus2 pilot placebo controlled RCT are being evaluated at present for the actions of intravenous mega dosage of Sodium ascorbate (60 g as well as 120 g) treatment (on renal outcomes besides vasopressor needs in patients with sepsis (ACTRN1262000651987 p; NCT04796636). |
||||
Proof is existent with regards to early stage sepsis, with renal microcirculatory impairment, due to inflammation as well as Oxidative stress, causes localized tissue hypoxia, along with mitochondrial impairment, thus starting a vicious cycle of cellular damage besides propagative AKI. Studies with regards to experimental sepsis suggested that anti-inflammatory besides antioxidant effects of NAC along with Vitamin C result in multi-organ protection from failure specifically on administration as a prophylactic agent or at the time of early stage. Nevertheless, these presumed advantages of NAC along with Vitamin C as visualized in animal studies have not got translated all the times in heterogeneous populations of patients with sepsis that have full development of multi-organ impairment on delivery at different time points of disease robustness over continued periods of time of treatment. As per preclinical studies in clinically applicable models of sepsis are the need of the hour for escalation of our insight of the modes of effects of antioxidants in case of sepsis, besides the determination of ideal dosages at a particular correct time period of sepsis (as in comparison in Tables 1 & 2), Vitamin C appears to have greater efficacy in contrast to NAC. These types of studies are necessary for a justified scientific basis for fashioning a larger RCT.
- Bagshaw SM, George C, DinuL, Bellomo R. A multi-center evaluation of the RIFLE criteria for early acute Kidney injury in critically ill patients. Nephrol Dial Transplant. 2007; 23: 1203-1210. PubMed: https://pubmed.ncbi.nlm.nih.gov/17962378/
- Bellomo RA, Kellum JA, Ronco C, Wald R, Martenson J, et al. Acute Kidney injury in sepsis. Intensive care Med. 2017; 43: 816-828. PubMed: https://pubmed.ncbi.nlm.nih.gov/28364303/
- Odutayo A, Wong CX, Farkoouh M, Altman DG, Hopewell S, et al. AKI and long term risk for cardiovascular and mortality. J Am Soc Nephrol. 28: 377-387. PubMed: https://pubmed.ncbi.nlm.nih.gov/27297949/
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. Surviving sepsis-campaign; International, Guidelines for management of sepsis and septic shock. Intensive care Med. 2017; 43: 304--377. PubMed: https://pubmed.ncbi.nlm.nih.gov/28101605/
- Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004; 351: 159-69. PubMed: https://pubmed.ncbi.nlm.nih.gov/15247356/
- Lerrole N, Nochy D, Guerot E, Bruneval P, Fagon JY, et al. Histopathology of septic shock induced Acute Kidney injury: apoptosis and leukocyte infiltration. Intensive Care Med. 2009; 36: 471-478. PubMed: https://pubmed.ncbi.nlm.nih.gov/19924395/
- Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, et al. Structure and function of Kidney in septic shock. A prospective controlled experimental study. Am J Respir Crit Care Med. 2016; 194: 692--700. PubMed: https://pubmed.ncbi.nlm.nih.gov/26967568/
- Langenberg C, Gobe G, Hood S, May CN, Bellomo R. Renal Histopathology during experimental Acute Kidney injury and recovery. Crit Care Med. 2014; 42: e58-e67. PubMed: https://pubmed.ncbi.nlm.nih.gov/24126439/
- Ma S, Evans R, Iguch IN, Tare M, Parkigton HC, et al. sepsis induced Acute Kidney injury: a disease of the microcirculation. Microcirculation. 2018; 26: e12483. PubMed: https://pubmed.ncbi.nlm.nih.gov/29908046/
- Calzavacca P, Evans RG, Bailey M, Bellomo R, May CN, et al. Renal cortical and medullary tissue perfusion and oxygenation in experimental septic Acute Kidney Injury. 2015; 43: e431-e439. PubMed: https://pubmed.ncbi.nlm.nih.gov/26181218/
- Lankadeva YR, Koska J, Evans RG, Bellomo R, May CN, et al. Urinary oxygenation as a surrogate marker of medullary oxygenation during Angiotensin II therapy in septic Acute Kidney injury. Crit Care Med. 2002; 46: e41-e48. PubMed: https://pubmed.ncbi.nlm.nih.gov/29077618/
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82: 47-95. PubMed: https://pubmed.ncbi.nlm.nih.gov/11773609/
- Kellum J, Prowle J. Paradigms of Acute Kidney injury in Intensive care setting. Nat Rev Nephrol. 2018; 14: 217--230. PubMed: https://pubmed.ncbi.nlm.nih.gov/29355173/
- Gomez H, Ince C, De Backer D, Pickkers P, Payen D, et al. A unified theory of sepsis-induced Acute Kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock. 2014; 3-11. PubMed: https://pubmed.ncbi.nlm.nih.gov/24346647/
- Ow CPC, Trask-Marino A, Betrie AH, Evans RG, et al. Targeting Oxidative stress in septic Acute Kidney injury: From theory to practice. J Clin Med. 2021; 10: 3798. PubMed: https://pubmed.ncbi.nlm.nih.gov/34501245/
- Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014; 20:1126-67. PubMed: https://pubmed.ncbi.nlm.nih.gov/23991888/
- Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol. 2017; 7: 373. PubMed: https://pubmed.ncbi.nlm.nih.gov/28890882/
- Herter JM, Rossaint J, Spiecker T, Zarbock A. Adhesion molecules involved in neutrophils recruitment during sepsis induced Acute Kidney injury. J Innate Immun. 2014; 6: 597-606. PubMed: https://pubmed.ncbi.nlm.nih.gov/24576991/
- Fujimi S, Ogura H, Tanaka H, Koh T, Hosotsubo H, et al. Activated polymorphonuclear leukocytes enhance production of leukocyte microparticles with increased adhesion molecules in patients with sepsis. J Trauma Acute Care Surg. 2002; 52: 443-448. PubMed: https://pubmed.ncbi.nlm.nih.gov/11901317/
- Ware LB, Fessel JB, May AK, Roberts LJ. Plasma biomarkers of Oxidant stress and development of organ failure in severe sepsis. Shock. 2001; 36: 12-17. PubMed: https://pubmed.ncbi.nlm.nih.gov/21372753/
- Ley K, Laudanna C, Cybulsky M, Noursharg S. Getting to the site of inflammation;the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007; 7: 678-689. PubMed: https://pubmed.ncbi.nlm.nih.gov/17717539/
- Cannon JG, Tompkins RG, Gelfand JA, Michie HR, Stanford GG, et al. Circulating interleukin-1 and as necrosis factor alpha in septic shock. J Infect Dis. 1990; 161: 79-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/2295861/
- Chen Y, Jin S, Teng X, Hu S, Zhang Z, et al. Hydrogen sulfide attenuates LPS-induced Acute Kidney injury by inhibiting inflammation and Oxidative stress. Oxidative Med Cell Longev. 2018; 6717212. PubMed: https://pubmed.ncbi.nlm.nih.gov/29636853/
- Babickova J, Klinkhammer BM, Buhl EM, Djudja JS, et al. Regardless of etiology progressive renal disease causes ultra structural and functional alterations of peritubular capillaries. Kidney Int. 2017; 91: 70-85. PubMed: https://pubmed.ncbi.nlm.nih.gov/27678159/
- McNeil E, Channon KM. The role of tetrahydrobiopterin in inflammation and cardiovascular disease. Thromb Haemost. 2012; 108: 832-839. PubMed: https://pubmed.ncbi.nlm.nih.gov/23052970/
- Alkaitis MS, Crabtree MJ. Re coupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling. Curr Heart Fail Rep. 2012; 9: 200-210. PubMed: https://pubmed.ncbi.nlm.nih.gov/22711313/
- Lankadeva YR, Singh RR, Moritz KM, Parkigton HC, Denton KM, et al. Renal dysfunction is associated with a reduced contribution of nitric oxide and enhanced vasoconstriction after a congenital Renal mass reduction in sheep. Circulation. 2015; 131: 280-288. PubMed: https://pubmed.ncbi.nlm.nih.gov/25369804/
- He X, Su F, Velissaris D, Selgado DR, de Souza Barros D, et al. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med. 2012; 40: 2833-2840. PubMed: https://pubmed.ncbi.nlm.nih.gov/22846780/
- Chelazzi C, Villa G, Mancinelli P, De Caudio AR, Adembri C. Glycocalyx and sepsis induced alterations in vascular permeability. Crit Care. 2015; 19: 26. PubMed: https://pubmed.ncbi.nlm.nih.gov/25887223/
- Butler MJ, Down CJ, Foster R, Satchell SC. The pathological relevance of increased glycocalyx permeability. Am J Pathol. 2020; 190: 742-751. PubMed: https://pubmed.ncbi.nlm.nih.gov/32035881/
- Ince C, Mayeux PR, Nguyen GT, Gomez H, Kellum JA, et al. The endothelium in sepsis. Shock. 2016; 45: 259-270. PubMed: https://pubmed.ncbi.nlm.nih.gov/26871664/
- Nelson A, BerkestedtI Schmidtchen A, Ljunggren L, Bodelson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008; 30: 623-627. PubMed: https://pubmed.ncbi.nlm.nih.gov/18497712/
- Yu WK, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, et al. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019; 23: 18. PubMed: https://pubmed.ncbi.nlm.nih.gov/30658667/
- Clapp BR, Hingorani AD, Kharbanda RK, Mohamed Ali V, Stephens JW, et al. Inflammation induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased Oxidative stress. Cardiovasc Res. 2004; 64: 172-178. PubMed: https://pubmed.ncbi.nlm.nih.gov/15364625/
- Souza ACP, Yuen PS, Star RA. Microparticles: markers and mediators of sepsis induced Microvascular dysfunction, immunosuppression, and AKI. Kidney Int. 2015; 87: 1100-1108. PubMed: https://pubmed.ncbi.nlm.nih.gov/25692956/
- Lankadeva YR, Okazaki N, Evans RG, Bellomo R, May CN. Renal medullary hypoxia:a newtherapeutic target for septic Acute Kidney injury. Seminal Nephrol. 2019; 39: 543-553. PubMed: https://pubmed.ncbi.nlm.nih.gov/31836037/
- Lankadeva YR, Cochrane AD, Marino B, Iguchi N, Hood SG, et al. Strategies that improve renal medullary oxygenation during experimental cardiopulmonary bypass. Kidney Int. 2019; 95: 1338-1346. PubMed: https://pubmed.ncbi.nlm.nih.gov/31005272/
- Heyman SN, Reichman J, Brezis M. Pathophysiology of radio contrast Nephropathy: a role for medullary hypoxia. Invest Radiol. 1990; 34: 685-691. PubMed: https://pubmed.ncbi.nlm.nih.gov/10548380/
- Ullah M, Basile DP. Role of renal hypoxia in the progression from Acute Kidney injury to Chronic Kidney Disease. Seminal Nephrol. 2019; 39: 567-580. PubMed: https://pubmed.ncbi.nlm.nih.gov/31836039/
- Evans RG, Smith DW, Lee CJ, Ngo JP, Gardiner BS. What makes the Kidney susceptible to hypoxia? Anat Rec. 2020; 303: 2544-2552. PubMed: https://pubmed.ncbi.nlm.nih.gov/31566903/
- Evans RG, Ince C, Joles JA, Smith DW, May CN, et al. Haemodynamic influence on Kidney oxygenation: Clinical implications of integrative physiology. Clin Exp Pharmacol Physiol. 2013; 40: 106-122. PubMed: https://pubmed.ncbi.nlm.nih.gov/23167537/
- Calzavacca P, Evans RG, Bailey M, Lankadeva YR, Bellomo R, et al. Long-term measurement of renal cortical and medullary tissue oxygenation and perfusion in unanesthetized sheep. Am J Physiol Integr Comp Physiol. 2015; 308: R832- R839. PubMed: https://pubmed.ncbi.nlm.nih.gov/25761701/
- Bonventre JV, Weiberg JM. Recent advances in the pathophysiology of ischaemic Acute Renal failure. J Am Soc Nephrol. 2003; 14: 2199-2210. PubMed: https://pubmed.ncbi.nlm.nih.gov/12874476/
- Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biochemical basis of homeostasis. Am J Physiol Physiol. 2008; 295: F1259- F1270. PubMed: https://pubmed.ncbi.nlm.nih.gov/18550645/
- Evans RG, Fitzergald S. Nitric oxide and superoxide in the renal medulla;a delicate balancing act. Curr Opin Nephrol Hypertens. 2005; 14: 9-15. PubMed: https://pubmed.ncbi.nlm.nih.gov/15586010/
- Lankadeva YR, Koska J, Evans RG, Bellomo R, May CN. Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic Acute Kidney injury. Kidney Int. 2006; 90: 100-108. PubMed: https://pubmed.ncbi.nlm.nih.gov/27165831/
- Lankadeva YR, Koska J, Iguchi N, Evans RG, Booth LC, et al. Effects of fluids bolus therapy on Renal perfusion, oxygenation and function in early experimental septic Kidney injury. Crit Care Med. 2019; 47: e36-e43. PubMed: https://pubmed.ncbi.nlm.nih.gov/30394921/
- Iguchi N, Lankadeva YR, Mori TA, Osawa EA, Cutuli SL, et al. Furosemide reverses medullary tissue i hypoxia in ovine experimental septic Kidney injury. Am J Physiol Integr Comp Physiol. 2019; 317: R232- R239. PubMed: https://pubmed.ncbi.nlm.nih.gov/31141418/
- Osawa EA, Cutuli S, L, Bitker L, Canet E, Ciocaari L, et al. Effect of Furosemide on urinary oxygenation in patients with septic shock. Blood Purif. 2019; 48: 336-345. PubMed: https://pubmed.ncbi.nlm.nih.gov/31336370/
- O’Connor PM, Kett MM, Anderson WP, Evans RG. Renal medullary tissue oxygenation is dependent on both cortical and medullary Blood flow. Am J Physiol Physiol. 2006; 291: F688- F694. PubMed: https://pubmed.ncbi.nlm.nih.gov/16219913/
- Haase VH. Hypoxia inducible factor 1 (HIF 1). Am J Physiol Physiol. 2006; 291: F271- F281. PubMed: https://pubmed.ncbi.nlm.nih.gov/16554418/
- Beck I, Weinmann R, Caro J. Characterization of hypoxia-responsive enhancer in the human erythropoietin gene shows presence of hypoxia-inducible 120-Kd nuclear DNA-binding protein in erythropoietin-producing and nonproducing cells. Blood. 1993; 82: 704-711. PubMed: https://pubmed.ncbi.nlm.nih.gov/8338939/
- Melillo G, Msso T, Sica A, Taylor LS, Cox GW, et al. A. hypoxia responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995; 182: 1683-1693. PubMed: https://pubmed.ncbi.nlm.nih.gov/7500013/
- Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J ClinInvestig. 2007; 117: 3810-3820. PubMed: https://pubmed.ncbi.nlm.nih.gov/18037992/
- Ince C, Milk EG. Micro Circulatory and mitochondrial hypoxia in sepsis shock and resuscitation. J Appl Physiol (1985). 2016; 120: 226-235. PubMed: https://pubmed.ncbi.nlm.nih.gov/26066826/
- Chande NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, et al. Mitochondrial Reactive oxygen species trigger hypoxia induced transcription. Proc Natl Acad Sci USA. 1988; 95: 11715-11720. PubMed: https://pubmed.ncbi.nlm.nih.gov/9751731/
- Sherz-Shoual R, Shvets E, Fass E, Shorer H, Gil L, et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of At4. EMBO J. 2007; 26: 1749-1760. PubMed: https://pubmed.ncbi.nlm.nih.gov/17347651/
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2016; 282: 2871-2879. PubMed: https://pubmed.ncbi.nlm.nih.gov/17132626/
- Nagar H, Piao S, Kim CS. Role of mitochondrial Oxidative stress in sepsis. Acute Crit Care. 2018; 33: 65-72. PubMed: https://pubmed.ncbi.nlm.nih.gov/31723865/
- Guzy GD, Schumacher PT. Oxygen sensing bymitochondria at Complex III: the paradox of Reactive oxygen species during hypoxia. Exp Physiol. 2006; 91: 807-819. PubMed: https://pubmed.ncbi.nlm.nih.gov/16857720/
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, et al. Reactive oxygen species generated at mitochondrial Complex IIIstabilizes Hypoxia inducible factor 1 alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2006; 275: 25130-25138. PubMed: https://pubmed.ncbi.nlm.nih.gov/10833514/
- Hernasanz-Agustin P, Ramos E, Navarro E, Parada E, Sanchez Lopez N, et al. Mitochondrial Complex In de activation is related to superoxide production in Acute hypoxia. Redox Biol. 2017; 12: 1040-1051. PubMed: https://pubmed.ncbi.nlm.nih.gov/28511347/
- Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, et al. RIPK3 promotes sepsis –induced Acute Kidney injury via mitochondrial dysfunction. JCI Insight. 2018; 3: e98411. PubMed: https://pubmed.ncbi.nlm.nih.gov/29875323/
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, et al. Association between mitochondrial dysfunctionand severity and outcome of septic shock. Lancet. 2002; 360: 219-223. PubMed: https://pubmed.ncbi.nlm.nih.gov/12133657/
- Plonikov EY, Pevzner IB, Zorova LD, Chemikov V, Prusov AN, et al. Mitochondrial damage and mitochondrial targeted antioxidants protection in LPS induced Acute Kidney injury. Antioxidants. 2019; 8: 176. PubMed: https://pubmed.ncbi.nlm.nih.gov/31197113/
- Pathak E, MacMillan-Crow LA, Mayeux PR. Role of mitochondrial oxidants In an in vitro model of sepsis –induced renal injury. J Pharmacol Exp Ther. 2012; 340: 192-201. PubMed: https://pubmed.ncbi.nlm.nih.gov/22011433/
- Ding Y, Zheng Y, Huang J, Peng W, Chen X, et al. UCP2ameliorates mitochondrial dysfunction, inflammation, Oxidative stress in lipopolysaccharides- induced Acute Kidney injury. Int Immunopharmacol. 2019; 71: 336-349. PubMed: https://pubmed.ncbi.nlm.nih.gov/30952098/
- Divakaruni AS, Brand M.The regulation and physiology of mitochondrial proton leak. Physiology. 2011; 26: 192-205. PubMed: https://pubmed.ncbi.nlm.nih.gov/21670165/
- Liu JX, Yang C, Zhang WH, Su HY, Liu ZJ, et al. Disturbance of mitochondrial dynamics and mitophagy in sepsis induced Acute Kidney injury. Life Sci. 2019; 235: 116828. PubMed: https://pubmed.ncbi.nlm.nih.gov/31479679/
- vander Slikke EC, Star BS, van Meurs M, Henningzrh Moser J, Bouma HR. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the Kidneys of patients with sepsis-AKI. Crit Care. 2021; 25: 36. PubMed: https://pubmed.ncbi.nlm.nih.gov/33494815/
- Yuan S, Akey CW. Apoptosome structure, assembly and procaspase activation. Structure. 2013; 21: 501-515. PubMed: https://pubmed.ncbi.nlm.nih.gov/23561633/
- Arouma OI, Halliwell B, Hoey BM, Butler J. The antioxidants action of N-Acetyl Cysteine: its reaction with hydrogen peroxide, hydroxyl radicals, super oxide and hypochlorous acid. Free Radical Biol Med. 1989; 6: 593-597. PubMed: https://pubmed.ncbi.nlm.nih.gov/2546864/
- Kharazmi A, Nielsen H, Schoetz PO. N-Acetyl Cysteine inhibits human neutrophils and monocyte chemotaxis and Oxidative metabolism. Int J Immunopharmacol. 1988; 10: 39-46. PubMed: https://pubmed.ncbi.nlm.nih.gov/3366508/
- Schmidt H, Schmidt W, Muller T, Bohrer H. The antioxidant action of N-Acetyl Cysteine attenuates endotoxin induced leukocyte to endothelial cells adhesion and macromolecular leakage in vivo. Crit Care Med. 1997; 25: 858-863. PubMed: https://pubmed.ncbi.nlm.nih.gov/9187607/
- Patterson RL, Galley HF, Webster NR. The effect of N-Acetyl Cysteine on nuclear factor κB activation, interleukin-6, interleukin-8 and intercellular cell adhesion molecule expression in patients with sepsis. Crit Care Med. 2003; 31: 2574-2578. PubMed: https://pubmed.ncbi.nlm.nih.gov/14605526/
- Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JCF, et al. Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med. 2004; 32: 342-349. PubMed: https://pubmed.ncbi.nlm.nih.gov/14758146/
- Hsu BG, Lee RP, Yang FL, Harn HJ, Chen HJ. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006; 79: 2010-2016. PubMed: https://pubmed.ncbi.nlm.nih.gov/16860347/
- Singer M, Deutschmann CS, Seymour CC, Shankar-Harl M, Annane D, et al. The third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016; 315: 801-810. PubMed: https://pubmed.ncbi.nlm.nih.gov/26903338/
- Vassilev D, Hauser B, Bracht H, Ivanyi Z, Schoaff M, et al. Systemic, pulmonary, and hepatosplanchnic effects of N-acetylcysteine during long-term porcine endotoxemia. Crit Care Med. 2004; 32: 525-532. PubMed: https://pubmed.ncbi.nlm.nih.gov/14758174/
- Szakmany T, Hauser B, Radermacher P. N-Acetyl Cysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012; CD006616. PubMed: https://pubmed.ncbi.nlm.nih.gov/22972094/
- Spapen HT, Diltoer MW, Nguyen GT, Hendrickckx I, Huyghens LP. Effects of N-Acetyl Cysteine on microalbuminuria and organ failure in acute severe sepsis: results of a pilot study. Chest. 2005; 127: 1413-1419. PubMed: https://pubmed.ncbi.nlm.nih.gov/15821223/
- Peake SL, Moran JL, Leppard PL. N-Acetyl Cysteine depresses cardiac performance in patients septic shock. Crit Care Med. 1996; 24: 1302-1310. PubMed: https://pubmed.ncbi.nlm.nih.gov/8706483/
- Molnar Z, Shearer E, Lowe D. N-Acetyl Cysteine treatment to prevent the multisystemorgan failure: a prospective randomized placebo controlled study. Crit Care Med. 1999; 27: 1100-1104. PubMed: https://pubmed.ncbi.nlm.nih.gov/10397212/
- Najaf A, Mojtahedzadeh M, Almadi KH, Abdollahi M, Mousavi M, et al. The immunological benefit of higher dose N-Acetyl Cysteine following mechanical ventilation in critically ill patients. DRU J Pharm Sci. 2014; 22: 57. PubMed: https://pubmed.ncbi.nlm.nih.gov/25027749/
- Padayatty SJ, Levine M. Vitamin C: the known and unknown of Goldilocks. Oral Dis. 22: 463-493. PubMed: https://pubmed.ncbi.nlm.nih.gov/26808119/
- Buetner GR, Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat Res. 1996; 145: 532-541. PubMed: https://pubmed.ncbi.nlm.nih.gov/8619018/
- Carcamo JM, Pedraza A, Borquez-Ojeda O, Golde DW. Vitamin C suppresses TNF-alpha induced NF kappa B activation, by inhibiting I kappa B alpha phosphorylation. Biochemistry. 2002; 41: 12995-13002. PubMed: https://pubmed.ncbi.nlm.nih.gov/12390026/
- Victor VV, Guayerbas N, Puerto M, Medina S, De la Fuente M. Ascorbic acid modulates in vivothe function of macrophagesfrom mice with endotoxic shock. Immunopharmacology. 2000; 46: 89-101. PubMed: https://pubmed.ncbi.nlm.nih.gov/10665783/
- Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents micro vascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol. 2001; 90: 795-803. PubMed: https://pubmed.ncbi.nlm.nih.gov/11181585/
- Mo SJ, Son EW, Rhee DK, Pyo S. Modulation of tnf-α induced icam1 expression, NO and H2O2production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharmacol Res. 2003; 26: 244-251. PubMed: https://pubmed.ncbi.nlm.nih.gov/12723939/
- Kim HJ, Lee SL, Lee DH, Smith D, Jo H, et al. Ascorbic acid synthesis due to L-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem Biophys Res Commun. 2006; 345: 1657-1662. PubMed: https://pubmed.ncbi.nlm.nih.gov/16737683/
- Ladurner A, Schmid CA, Schachner D, Atanasov A, Werner ER, et al. Ascorbate stimulates endothelial nitric oxide synthase activity by rapid Modulation of its phosphorylation status. Free Radical Biol Med. 2012; 52: 2082-2090. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3377995/
- Schneider MP, Delles C, Schmidt BM, Oehmer S, Schwartz TK, et al. superoxide scavenging effects of N-Acetyl Cysteine and Vitamin C in subject with essential hypertension. Am J Hypertens. 2005; 18: 1111-1117. PubMed: https://pubmed.ncbi.nlm.nih.gov/16109326/
- Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate dependent vasopressor synthesis: a rationale for Vitamin C administration in severe sepsis and septic shock? Crit Care. 2015; 19: 418. PubMed: https://pubmed.ncbi.nlm.nih.gov/26612352/
- Carr AC, Rosengrave PC, Bayer D, Chambers S, Mehrtens J, et al. Hypovitaminosis C and deficiency in critically ill patients despite recommended Ther enteral and parenteral intake. Crit Care. 2017; 21: 300. PubMed: https://pubmed.ncbi.nlm.nih.gov/29228951/
- May CN, Bellomo R, Lankadeva YR. Therapeutic potential of megadose Vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br J Pharmacol. 2021; 178: 3864-3868. PubMed: https://pubmed.ncbi.nlm.nih.gov/34061355/
- Wilson JX. Evaluation of Vitamin C for adjuvant sepsis therapy. Anti Oxid Redox Signal. 2013; 19: 2129-2140. PubMed: https://pubmed.ncbi.nlm.nih.gov/23682970/
- Zhou G, Kamenos G, Pendem D, Wilson JX, Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture induced septic peritonitis. Am J Physiol Integr Comp Physiol. 2012; 302: R409- R416. PubMed: https://pubmed.ncbi.nlm.nih.gov/22116513/
- Tym lK, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine nucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by Ascorbate through an endothelial nitric oxide synthase dependent mechanism. Crit Care Med. 2008; 36: 2355-2362. PubMed: https://pubmed.ncbi.nlm.nih.gov/18596627/
- Wu F, Wilson JX, Tyml K. Ascorbate protects against impaired arterioral constriction in sepsis byinhibiting induciblenitric oxide synthase expression. Free Radical Biol Med. 2004; 37: 1282-1289. PubMed: https://pubmed.ncbi.nlm.nih.gov/15451067/
- Fowler AA, Syed A, Knowlson S, Sculthorpe R, Farhin D, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014; 12: 32. PubMed: https://pubmed.ncbi.nlm.nih.gov/24484547/
- Zabet MH, Mohammedi M, Ramezani M, Khalili H. Effect of high dose ascorbic acid on vasopressor requirements in septic shock. J Respharm Pract. 2016; 5: 94-100. PubMed: https://pubmed.ncbi.nlm.nih.gov/27162802/
- Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017; 151: 1229-1238. PubMed: https://pubmed.ncbi.nlm.nih.gov/27940189/
- Fuji T, Luethi N, Young PJ, Frei DR, Eastwood GM, et al. Effect of Vitamin C, Hydrocortisone, and thiamine vs Hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: VITAMINS randomized Clinical trial. JAMA. 2020; 323: 423-431. PubMed: https://pubmed.ncbi.nlm.nih.gov/31950979/
- Moscowitz A, Huang DT, Hou FC, Gong J, Doshi B, et al. Effects of ascorbic acid, corticosteroids and thiamine on organ injury in septic shock. The ACTS randomized Clinical trial. JAMA. 2020; 324: 642-650. PubMed: https://pubmed.ncbi.nlm.nih.gov/32809003/
- Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, et al. Combination treatment of Vitamin C and thiamine for septic shock; a multicentre, double blinded randomized controlled study. Intensive Care Med. 2020; 46: 2015-2025. PubMed: https://pubmed.ncbi.nlm.nih.gov/32780166/
- Fowler AA. Truwit JD, Hie RD, Morris PE, de Wilde C, et al. Effect of Vitamin C organ failure and biomarkers of inflammation and Vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS ALI randomized Clinical trial. JAMA. 2019; 322: 1261-1270. PubMed: https://pubmed.ncbi.nlm.nih.gov/31573637/
- Subramanium VS, Sabui S, Moradi H, Marchant JS, Said HM. Tumor necrosis factor alpha reduces intestinal Vitamin C uptake; arole for NFκB mediated signaling. Am J Physiol Gastroenterol Liver Physiol. 2018; 315: G241-G248. PubMed: https://pubmed.ncbi.nlm.nih.gov/29631379/
- Yahase F, Fujii, Naorungroj T, Belletti A. Luethi N, et al. Harm of IV high dose Vitamin C the ray in adult patients: a scoping review. Crit Care Med. 2021; 48: e620- e628. PubMed: https://pubmed.ncbi.nlm.nih.gov/32404636/
- Lankadeva YR, Peiris RM, Okazaki N, BirchallI E, Trask-Marino A, et al. Reversal of the pathophysiological responses to gram negative sepsis by mega dose Vitamin C. Crit Care Med. 2021; 49: e179-e190. PubMed: https://pubmed.ncbi.nlm.nih.gov/33239507/
- Kaur KK, Allahbadi GN, Singh M. Have we done justice to Linus Pauling’s discovery in 1970s with Regards to utilization of Vitamin C for prevention of acute respiratory illnesses, besides role of Vitamin C in current COVID19 Epidemic - A Comprehenive review. Acta Sci Nutr Health. 2002; 6: 1-14.