More Information
Submitted: April 05, 2021 | Approved: April 26, 2021 | Published: April 27, 2021
How to cite this article: Yang Q, Huang W, Zeng X, Zheng J, Chen W, et al. Nonlinear relationship between blood glucose and 30-day mortality in critical patients with acute kidney injury: A retrospective cohort study. J Clini Nephrol. 2021; 5: 042-046.
DOI: 10.29328/journal.jcn.1001072
Copyright License: © 2021 Yang Q, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abbreviations: BPM: Beat Per Minute; MAP: Mean Arterial Pressure; RRT: Renal Replace Treatment; WBC: White Blood Count; SAPS, Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; CHF: Congestive Heart Failure; AF: Atrial Fibrillation; COPD: Chronic Obstructive Pulmonary Diseases
Nonlinear relationship between blood glucose and 30-day mortality in critical patients with acute kidney injury: A retrospective cohort study
Qilin Yang1#, Weichao Huang2#, Xiaomei Zeng2, Jiezhao Zheng1, Weixiao Chen1 and Deliang Wen1*
1Department of Critical Care, The Second Affiliated Hospital of Guangzhou Medical University, No. 250 Changgang East Road, Haizhu District, Guangzhou, China
2Guangzhou Medical University, Guangzhou, 511436, Guangdong, China
#Contributed equally
*Address for Correspondence: Deliang Wen, Department of Critical Care, the Second Affiliated Hospital of Guangzhou Medical University, No. 250 Changgang East Road, Haizhu District, Guangzhou, China, Tel: 18927588355; Email: [email protected]
Background: Acute kidney injury (AKI) is a major health problem affecting millions of people worldwide. Effective preventative and therapeutic treatments remain to be produced. We aim to determine the association between blood glucose and mortality in critical patients with AKI.
Method: This cohort study included 18,703 patients with AKI. The exposure of interest was baseline blood glucose. The outcome was 30-day mortality. Multivariable Cox regression analyses and smooth curve fitting were adopted to assess the independent association between blood glucose and 30-day mortality.
Results: We identified 18,703 consecutive individuals with AKI. The average age of the participants was 66.8 ± 16.0 years, and about 42.7% of them were female. The overall 30-day mortality was 16.9%. Through the multivariate COX regression model and smooth curve fitting, we observed that the correlation between blood glucose and 30-day mortality is nonlinear. An inflection point was found at about 5.93 mmol/L. On the left side of inflection point, the effect size was 0.81 (HR: 0.81, 95% CI 0.74-0.89, p < 0.001). On the right side of inflection point, the effect size was 1.02 (HR: 1.02,95% CI 1.01-1.03, p < 0.001).
Conclusion: Our study suggested that, among patients with AKI, there was a nonlinearity relationship between blood glucose and mortality in patients with AKI. The optimal of blood glucose associated with the lowest risk of 30-day mortality was around 5.93 mmol/L.
Acute kidney injury (AKI) is or health problem affecting millions of people worldwide, leading to decreased survival, underlying chronic kidney disease (CKD) progression, and new CKD onset occasionally [1]. Effective preventative and therapeutic treatments remain to be produced [2].
Previous study showed an increase in the blood glucose levels beyond normal values is associated with an increase in the incidence of AKI [3,4]. However, researches have known far less about the relationship between blood glucose and mortality in critical patients with AKI. In addition, our previous study indicated an antihyperglycemic agent, metformin may be associated with reduced risk-adjusted mortality in patients with AKI [5]. Therefore, we conducted a retrospective cohort study to determine the association between blood glucose and mortality in critical patients with AKI.
We conducted a retrospective cohort study and enrolled critical patients with AKI from the Medical Information Mart for Intensive Care (MIMIC)-III (version 1.4). MIMIC-III is a real-world clinical database containing more than 60,000 intensive care unit (ICU) admissions at Beth Israel Deaconess Medical Center between 2001 and 2012 [6]. Qilin Yang, one of the authors, obtained approval to use the database (certification number 7634793) [5]. All reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [7].
Study population
Adult patients (older than 18 years) in the MIMIC-III who fullfed the definition of AKI within 48 hours after ICU admission were eligible for inclusion. AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria. KDIGO criteria include [8], increase in serum creatinine (SCr) ≥ 1.5 times baseline within the prior 7 days, ≥ 0.3 mg/dL increase in SCr within 48 h, or urine volume < 0.5 mL/kg/h for at least 6 h. The lowest of the SCr values available within 7 days before admission was used as the baseline SCr [9]. When SCr prior to admission was not available, the first SCr measured on ICU admission was used as the baseline SCr [10]. For patients with recurrent ICU admissions, only the first ICU admission was considered.
Variable extraction
Blood glucose: We obtained baseline blood glucose as the first glucose within 24 h after ICU admission in MIMIC-III database.
Covariates: We included the following variables based on published literature and our clinical experience: demographic characteristics, and baseline heart rate, mean arterial pressure (MAP), SPO2, white blood cell (WBC) count, hemoglobin, platelet count, serum creatinine (SCr), simplified acute physiology score (SAPS) II score, ventilator use in first day, vasopressor use in first day, renal replacement therapy (RRT) use in first day, and comorbidities (congestive heart failure liver disease, coronary heart disease stroke, malignancy, diabetes). Vasopressors included norepinephrine, epinephrine, phenylephrine, vasopressin, dopamine, dobutamine, and isoprenaline.
Outcome: The outcome was 30-day mortality.
Statistical analysis
Descriptive analysis was performed for all patients. Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as mean and standard deviation (SD) for normal distributions or median and interquartile range for skewed distributions. We used the chi-square test, one-way ANOVA, and Kruskal-Wallis test for the comparison of categorical, normally distributed, and non-normally distributed continuous variables, respectively.
Multivariable Cox regression analyses and smooth curve fitting were adopted to assess the independent association between blood glucose and 30-day mortality. To examine the nonlinear association between blood glucose levels and 30-day mortality, we further applied a two-piecewise linear regression model using a smoothing curve. We conducted a loglikelihood ratio test comparing the one-line linear regression model with the two-piecewise linear model. Survival curves were plotted by Kaplan–Meier and log-rank analyses.
All the analyses were performed with the statistical software packages R 3.3.2 (http://www.r-project.org, The R Foundation) and Free Statistics software versions 1.1. A two-tailed test was performed and p < 0.05 was considered statistically significant.
Baseline characteristics of participants
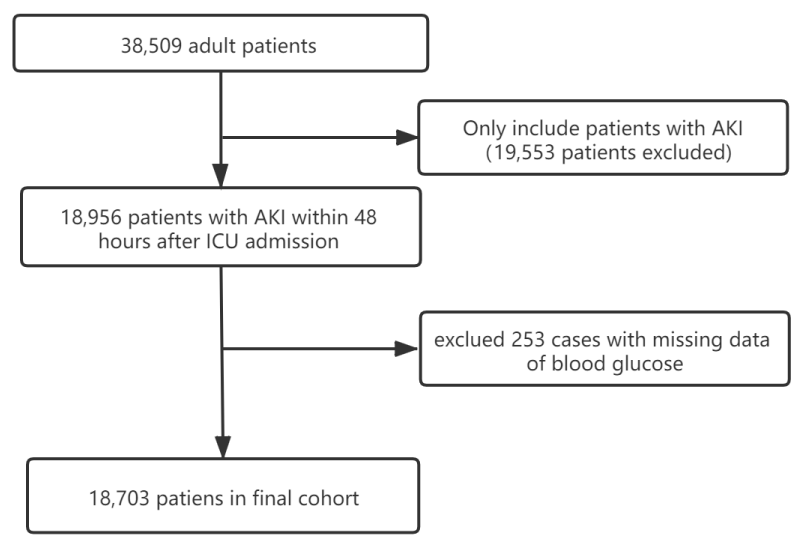
We identified 18,703 consecutive individuals with AKI according to the KDIGO definition (Figure 1). Baseline characteristics of selected participants according to quartiles of blood glucose are shown in table 1. In general, the average age of the participants was 66.8 ± 16.0 years old, and about 42.7 of them were female. The overall 30-day mortality was 16.9%.
Figure 1: Flow chart of patient disposition.
| Table 1: Baseline characteristics of participants. | ||||||
| Covariates | Baseline blood glucose mmol/L) | |||||
| All patients | Q1 ≤ 6.11 | Q2 6.11-7.44 | Q3 7.44-9.28 | Q4 ≥ 9.28 | p - value | |
| (n = 18703) | (n = 4675) | (n = 4632) | (n = 4633) | (n = 4763) | ||
| Age (years) | 66.8 ± 16.0 | 65.4 ± 17.6 | 67.0 ± 16.2 | 67.5 ± 15.1 | 67.4 ± 14.9 | < 0.001 |
| Sex, n (%) | 0.03 | |||||
| Female | 7987 (42.7) | 2021 (43.2) | 1908 (41.2) | 1958 (42.3) | 2100 (44.1) | |
| Male | 10716 (57.3) | 2654 (56.8) | 2724 (58.8) | 2675 (57.7) | 2663 (55.9) | |
| Heart rate (bpm) | 88.1 ± 19.2 | 87.3 ± 19.9 | 87.5 ± 18.7 | 87.5 ± 18.4 | 90.2 ± 19.6 | < 0.001 |
| MAP (mmHg) | 81.7 ± 18.3 | 80.6 ± 18.1 | 81.6 ± 18.2 | 82.5 ± 17.9 | 82.1 ± 19.0 | < 0.001 |
| SPO2 (%) | 97.5 ± 4.4 | 97.4 ± 4.1 | 97.6 ± 3.8 | 97.7 ± 4.2 | 97.2 ± 5.1 | < 0.001 |
| WBC (×109) | 12.8 ± 9.2 | 11.7 ± 8.4 | 12.3 ± 9.1 | 13.1 ± 10.2 | 14.0 ± 8.8 | < 0.001 |
| Hemoglobin (g/L) | 10.8 ± 2.2 | 10.7 ± 2.0 | 10.8 ± 2.1 | 10.7 ± 2.1 | 10.9 ± 2.6 | 0.012 |
| Potassium (mmol/L) | 4.2 ± 0.8 | 4.1 ± 0.7 | 4.2 ± 0.7 | 4.3 ± 0.8 | 4.4 ± 0.9 | < 0.001 |
| Sodium (mmol/L) | 138.1 ± 4.8 | 138.5 ± 5.0 | 138.3 ± 4.6 | 138.0 ± 4.5 | 137.8 ± 5.2 | < 0.001 |
| Platelet (×1012) | 192.0 (139.0, 258.0) | 189.0 (135.0, 57.0) | 190.0 (137.5,254.5) | 190.0 (140.0, 253.0) | 200.0 (143.0, 70.0) | < 0.001 |
| Creatinine (mmoI/L) | 0.9 (0.7, 1.4) | 0.9 (0.7, 1.5) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | 1.0 (0.8, 1.5) | < 0.001 |
| CHF, n (%) | 5391 (28.8) | 1294 (27.7) | 1286 (27.8) | 1282 (27.7) | 1529 (32.1) | < 0.001 |
| AF, n (%) | 5876 (31.4) | 1410 (30.2) | 1504 (32.5) | 1525 (32.9) | 1437 (30.2) | 0.003 |
| Liver disease, n (%) | 1117 ( 6.0) | 375 (8) | 249 (5.4) | 208 (4.5) | 285 (6) | < 0.001 |
| COPD, n (%) | 2384 (12.7) | 588 (12.6) | 578 (12.5) | 580 (12.5) | 638 (13.4) | 0.487 |
| Caridiovascular disease, (%) | 6435 (34.4) | 1395 (29.8) | 1623 (35) | 1743 (37.6) | 1674 (35.1) | < 0.001 |
| Stroke, n (%) | 1789 ( 9.6) | 361 (7.7) | 463 (10.0) | 463 (10.0) | 502 (10.5) | < 0.001 |
| Malignancy, n (%) | 3091 (16.5) | 822 (17.6) | 816 (17.6) | 750 (16.2) | 703 (14.8) | < 0.001 |
| Diabetes | 5398 (28.9) | 867 (18.5) | 878 (19) | 1324 (28.6) | 2329 (48.9) | < 0.001 |
| Vasopressor use, n (%) | 7538 (40.3) | 1638 (35) | 1759 (38) | 2032 (43.9) | 2109 (44.3) | < 0.001 |
| Sedative use, n (%) | 10438 (55.8) | 2265 (48.4) | 2519 (54.4) | 2819 (60.8) | 2835 (59.5) | < 0.001 |
| Ventilator use, n (%) | 11373 (60.8) | 2452 (52.4) | 2688 (58) | 3042 (65.7) | 3191 (67.0) | < 0.001 |
| RRT, n (%) | 504 ( 2.7) | 153 (3.3) | 103 (2.2) | 97 (2.1) | 151 (3.2) | < 0.001 |
| SOFA score | 4.8 ± 3.3 | 4.8 ± 3.5 | 4.5 ± 3.0 | 4.7 ± 3.1 | 5.3 ± 3.4 | < 0.001 |
| SAPS II score | 38.3 ± 14.7 | 37.6 ± 15.3 | 36.8 ± 13.8 | 37.8 ± 14.0 | 40.8 ± 15.2 | < 0.001 |
| BPM: Beat Per Minute; MAP: Mean Arterial Pressure; RRT: Renal Replace Treatment; WBC: White Blood Count; SAPS, Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; CHF: Congestive Heart Failure; AF: Atrial Fibrillation; COPD: Chronic Obstructive Pulmonary Diseases | ||||||
Relationship between the blood glucose and 30-day mortality in AKI patients
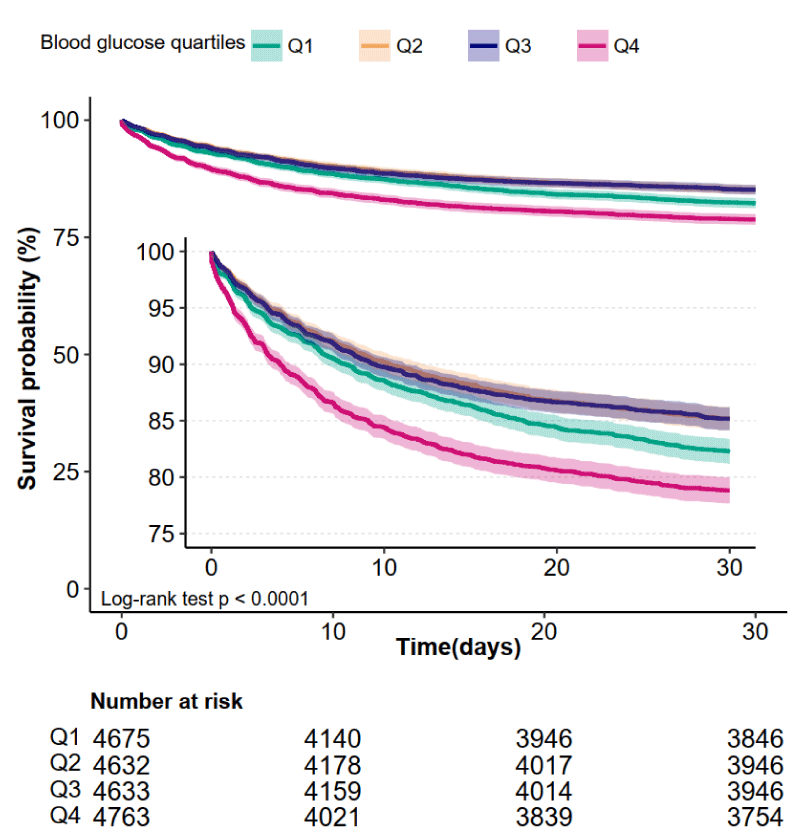
Kaplan-Meier curve showed there was lower mortality in patients in Q2 (6.11-7.44 mmol/L) and Q3 (7.44-9.28 mmol/L) groups (Log-rank test: p < 0.0001, Figure 2). The results of univariate and multivariate COX regression model are shown in table 2. In fully adjusted model (Adjusted for all covariates in table 1), a 1 mmol/L increment in blood glucose was associated with 1% higher 28-day mortality (HR = 1.01; 95% CI, 1.01, 1.02, p = 0.012, Table 2). For the purpose of sensitivity analysis, we also handled blood glucose as a categorical variable (quartiles) and found p for trend was 0.122, Table 2).
Figure 2: Kaplan-Meier Survival Curves for day 30 of AKI patients.
| Table 2: Relationship between blood glucose and 30-day mortality. | ||||||
| Exposure | Non-adjusted model | Minimally adjusted model | Fully adjusted model | |||
| HR (95% CI) | p - value | HR (95% CI) | p - value | HR (95% CI) | p - value | |
| Blood glucose mmol/L | 1.03 (1.02,1.04) | < 0.001 | 1.03 (1.02,1.04) | < 0.001 | 1.01 (1.002,1.02) | 0.012 |
| Blood glucose quartiles | ||||||
| Q1( ≤ 6.11 mmol/L) | Reference | Reference | Reference | |||
| Q2 6.11-7.44 mmol/L) | 0.82 (0.74,0.91) | < 0.001 | 0.8 (0.73,0.89) | < 0.001 | 0.94 (0.85,1.05) | 0.27 |
| Q3 7.44-9.28 mmol/L) | 0.83 (0.75,0.91) | < 0.001 | 0.8 (0.73,0.89) | < 0.001 | 0.89 (0.8,0.99) | 0.025 |
| Q4 ≥ 9.28 mmol/L) | 1.24 (1.13,1.36) | < 0.001 | 1.21 (1.11,1.33) | < 0.001 | 1.09 (0.99,1.20) | 0.079 |
| p for trend | < 0.001 | < 0.001 | 0.122 | |||
| Non-adjusted: no covariates were adjusted; Minimally adjusted model: Adjusted for age, gender; Fully adjusted model: Adjusted for all covariates in table 1. | ||||||
The nonlinear relationship between blood glucose and 30-day mortality
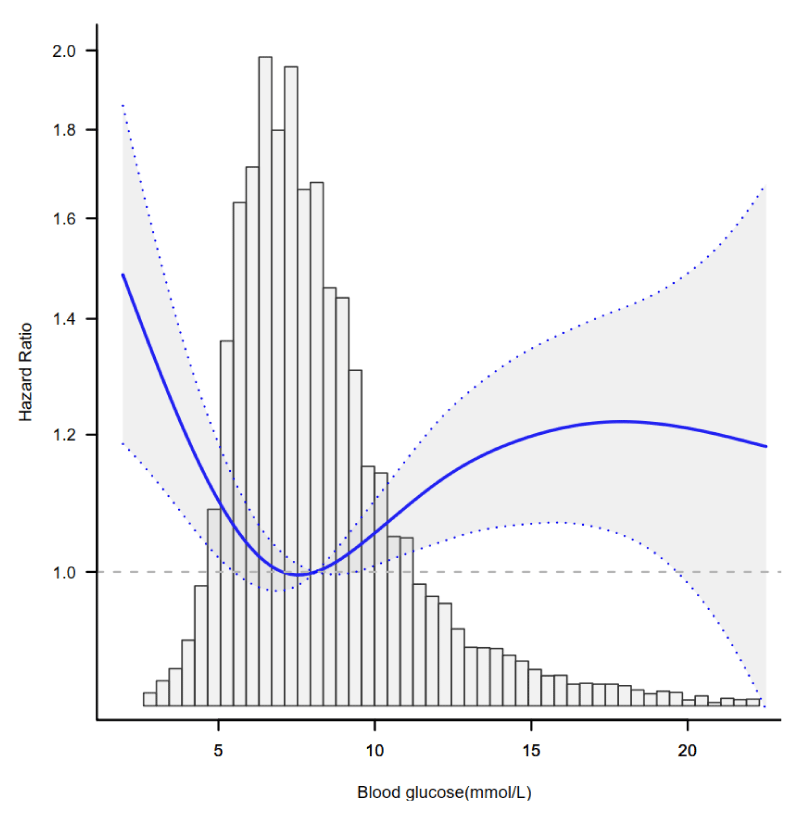
Through the multivariate COX regression model and smooth curve fitting, we observed that the correlation between blood glucose and 30-day mortality is nonlinear (Figure 3). Data were fit to a piecewise multivariate COX regression model to fits two different slopes. In our study, the p for log-likelihood ratio test was less than 0.001 (Table 3), we thus used two-piecewise model to fitting the link between blood glucose and 30-day mortality. We found an inflection point at about 5.93 mmol/L. On the left side of inflection point, the effect size was 0.81 (HR: 0.81, 95% CI 0.74-0.89, p < 0.001) On the right side of inflection point, the effect size was 1.02 (HR: 1.02,95% CI 1.01-1.03, p < 0.001).
Figure 3: The nonlinear relationship between blood glucose and 30-day mortality. Adjusted for all covariates in table 1.
| Table 3: The nonlinear relationship between blood glucose and 30-day mortality. | |||
| Threshold of driving pressure | HR | 95% CI | p - value |
| < 5.93 mmol/L | 0.81 | 0.74, 0.89 | < 0.0001 |
| ≥ 5.93 mmol/L Likelihood Ratio test |
1.02 | 1.01, 1.03 | < 0.0001 < 0.001 |
| Adjusted for all covariates in table 1. | |||
In this observational retrospective cohort study, we examined the optimal of blood glucose associated with 30-day mortality in critical patients with AKI using MIMC-III database. We found nonlinear association between blood glucose with 30-day mortality in these patients. The correlations between blood glucose and 30-day mortality of critical patients with AKI were totally different below and above the inflection point which was 5.93 mmol/L. Blood glucose, as assessed at baseline, was negatively associated below the 5.93 mmol/L, and it was positively associated with 30-day mortality of AKI patients above the 5.93 mmol/L. The optimal of blood glucose associated with the lowest risk of 30-day mortality was around 5.93 mmol/L.
The explanations for the nonlinear relationship between glucose level and mortality in critical patients with AKI have not been well established. Hypoglycemia increases risk of death in critically ill patients [11]. Hypoglycemia may induce sympathetic adrenal activation, abnormal cardiac repolarization, thrombosis, inflammation and vasoconstriction, which may further lead to adverse reactions [12,13]. Slightly elevated blood glucose may be an evolutionarily conserver adaptive strategy in nature that allows the host to survive during illness [14]. However, excessively high blood glucose may lead to the increases the inflammation reaction and cause immunosuppression, endothelial cell dysfunction, nervous system injury, oxidative stress [15,16]. Clinical studies also suggested hyperglycemia is strongly associated with increased coronary intervention associated AKI and in-hospital mortality [4].
There are exceedingly few published data on blood glucose and mortality in AKI. However, several previous studies have demonstrated the nonlinear relationship between fasting glucose level and adverse outcomes, such as the risk of incident atherosclerotic cardiovascular diseases [17] and all-cause mortality by age in diabetes [18]. Consistent with our study, several studies found, the optimal range of blood glucose levels associated with the lowest risk of all-cause mortality was 5.27–6.94 mmol/L [19,20]. In critical patients, among patients with sepsis, based on a meta-analysis, there was a nonlinear relationship between blood glucose with blood glucose level at 8.06 to 8.61 mmol/L corresponding to lowest mortality. Since our study enrolled only AKI patients, the optimal blood glucose associated with the lowest mortality (around 5.93 mmol/L) could be lower than that reported in previous studies in sepsis. Our study further extended this nonlinear relationship in critical patients with AKI.
Our research has the following shortcomings and needs attention: First, residual confounders such as reason of AKI, smoking status and alcohol use potentially exist, as with all retrospective analyses. We adjusted for all possible confounders as we can. Second, our findings can be only generalized to critical patients with AKI only, and the correlation of glucose on mortality may be different in other patients. Third, previous glycemic control/baseline glucose and Glycosylated haemoglobin prior to ICU admission were not in our analysis, however we adjusted history of diabetes. Finally, the causes of death were not recorded in the MIMIC-III database, we could not conduct a competing risk analysis.
In summary, our study suggested that, among patients with AKI, there was a nonlinear relationship between blood glucose and mortality in patients with AKI. The optimal of blood glucose associated with the lowest risk of 30-day mortality was around 5.93 mmol/L.
Availability of data and materials
Data in the article can be obtained from the MIMIC-III database (https://mimic.physionet.org/)
Funding
This article is fully funded by <
Authors’ contributions
Qi-lin Yang and Weichao Huang conducted data analysis and wrote the manuscript. Xiaomei Zeng conducted data analysis. Jie-zhao Zheng conducted data clean. Wei-xiao Chen conducted data collection and data interpretation. Deliang Wen designed the study and reviewed the manuscript.
All authors read and approved the manuscript for publication.
- Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012; 81: 819-825. PubMed: https://pubmed.ncbi.nlm.nih.gov/21975865/
- Moore PK, Hsu RK, Liu KD: Management of Acute Kidney Injury: Core Curriculum 2018. Am J Kidney Dis. 2018; 72: 136-148. PubMed: https://pubmed.ncbi.nlm.nih.gov/29478864/
- Azevedo J, Azevedo R, Lucena L, Costa N, Sousa W. Does intensive insulin therapy really reduce the incidence of acute renal injury in critically ill patients? An analysis using the rifle criteria. Crit Care. 2009; 13(Suppl 3): P37. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4085437/
- Lin K, Shang X, Guo Y, Zhu P, Wu Z, et al. Association of Preprocedural Hyperglycemia With Contrast-Induced Acute Kidney Injury and Poor Outcomes After Emergency Percutaneous Coronary Intervention. Angiology. 2018, 69: 770-778. PubMed: https://pubmed.ncbi.nlm.nih.gov/29463106/
- Yang Q, Zheng J, Wen D, Chen X, Chen W, et al. Association between metformin use on admission and outcomes in intensive care unit patients with acute kidney injury and type 2 diabetes: A retrospective cohort study. J Crit Care. 2021; 62: 206-211. PubMed: https://pubmed.ncbi.nlm.nih.gov/33422811/
- Johnson AEW, Pollard TJ, Lu S, Lehman LWH, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data. 2016; 3: 160035. PubMed: https://pubmed.ncbi.nlm.nih.gov/27219127/
- Elm EV, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007; 335: 806-808. PubMed: https://pubmed.ncbi.nlm.nih.gov/17947786/
- Levin A, Stevens PE, Bilous RW. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3: 1.
- Zhao G, Xu C, Ying J, Lü W, Hong G, et al. Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. Crit Care. 2020; 24.
- Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015; 64: 531-537. PubMed: https://pubmed.ncbi.nlm.nih.gov/25631669/
- Sekitoleko R, Jacob ST, Banura P, Pinkerton R, Meya DB, et al. Hypoglycemia at admission is associated with inhospital mortality in Ugandan patients with severe sepsis. Crit Care Med. 2011; 39: 2271-2276. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3730257/
- Wang W, Chen W, Liu Y, Li L, Li S, et al. Blood Glucose Levels and Mortality in Patients With Sepsis: Dose–Response Analysis of Observational Studies. J Intensive Care Med. 2021; 36: 182-190. PubMed: https://pubmed.ncbi.nlm.nih.gov/31746263/
- Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, et al. Severe hypoglycemia and risks of vascular events and death. New Engl J Med. 2010; 363: 1410-1418.
- Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! In. 2013; 41: e93-e94. PubMed: https://pubmed.ncbi.nlm.nih.gov/23685597/
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004: 27: 553-591. PubMed: https://pubmed.ncbi.nlm.nih.gov/14747243/
- Schmoch T, Uhle F, Siegler BH, Fleming T, Morgenstern J, et al. The Glyoxalase System and Methylglyoxal-Derived Carbonyl Stress in Sepsis: Glycotoxic Aspects of Sepsis Pathophysiology. Int J Mol Sci. 2017; 18: 657. PubMed: https://pubmed.ncbi.nlm.nih.gov/28304355/
- Park C, Guallar E, Linton JA, Lee D, Jang Y, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013; 36: 1988-1993. PubMed: https://pubmed.ncbi.nlm.nih.gov/23404299/
- Yi S, Park S, Lee Y, Balkau B, Yi J. Fasting Glucose and All-Cause Mortality by Age in Diabetes: A Prospective Cohort Study. Diabetes Care. 2018; 41: 623-626. PubMed: https://pubmed.ncbi.nlm.nih.gov/29301823/
- Lee JH, Han K, Huh JH. The sweet spot: fasting glucose, cardiovascular disease, and mortality in older adults with diabetes: a nationwide population-based study. Cardiovasc diabetol. 2020; 19: 44.
- Lu J, He J, Li M, Tang X, Hu R, et al. Predictive Value of Fasting Glucose, Postload Glucose, and Hemoglobin A(1c) on Risk of Diabetes and Complications in Chinese Adults. Diabetes Care. 2019; 42: 1539-1548. PubMed: https://pubmed.ncbi.nlm.nih.gov/31152120/