More Information
Submitted: March 30, 2021 | Approved: April 21, 2021 | Published: April 22, 2021
How to cite this article: Yassir Z, Aya S, Taoufiq A, Mounia A, El Kabbaj D. Diffuse alveolar hemorrhage: Unusual presentation of systemic lupus erythematosus. J Clini Nephrol. 2021; 5: 031-033.
DOI: 10.29328/journal.jcn.1001070
Copyright License: © 2021 Yassir Z, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Alveolar; Hemorrhage; Lupus; Nephritis
Diffuse alveolar hemorrhage: Unusual presentation of systemic lupus erythematosus
Zajjari Yassir*, Sobhi Aya, Aatif Taoufiq, Azizi Mounia and El Kabbaj Driss
Department of Nephrology-Dialysis, Military Hospital Mohammed V, Hay Ryad BP 10100 Rabat, Morocco
*Address for Correspondence: Dr. Yassir Zajjari, Department of Nephrology-Dialysis, Military Hospital Mohammed V, Hay Ryad BP 10100 Rabat, Morocco, Tel: 00212632372220; Email: [email protected]
Diffuse alveolar hemorrhage (DAH) is a rare complication of systemic lupus erythematosus (SLE) and carries a high mortality.
It was first described by Osler in 1904 as the most devastating pulmonary complication of SLE.
We describe a case of a 23-year-old girl recently diagnosed with SLE associated by a class III nephritis treated with oral corticoids and mycophenolate mofetil who developed a Diffuse Alveolar Hemorrhage DAH a few days later. The early diagnosis and the aggressive therapy allowed us to have a favorable outcome.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by diverse organs involvement. Thus, it may involve skin, joint, kidney, lung, heart, vascular system, gastrointestinal tract, and nervous system. Pulmonary involvement in SLE can occur in 50% – 70% of affected patients [1–3]. The pleuro-pulmonary presentations include pleuritis, acute lupus pneumonitis, lymphoid interstitial pneumonia, pulmonary embolism with antiphospholipid syndrome, pulmonary hypertension, shrinking lung syndrome, pulmonary nodules, bronchiolitis obliterans, infections, diaphragmatic involvement and diffuse alveolar hemorrhage (DAH) [1,4,5].
DAH is a rare fatal complication with reported frequency ranging from 1% to 5.4% of lupus series [6,7]. It was first described by Osler in 1904, as the most devastating pulmonary manifestation of SLE [8].
In this paper, we present a case of a patient recently diagnosed with SLE associated with active lupus proliferative nephritis treated with oral corticoids and immunosuppressive therapy who developed DAH a few days later.
A 23-year-old woman presented to our hospital with three months history of fatigue, loss of appetite, photosensitivity and polyarthralgia. On examination, she weighed 60 kg with height of 152 cm. She was afebrile, pale, with pulse rate of 98/min and blood pressure of 150/70 mm Hg. She also had a malar rash. Her cardiovascular, abdominal and respiratory system examinations were normal. Urinalysis showed 3+ red blood cells and 2+protein. The amount of proteinuria was 2 g/day. Urine culture was sterile. She has anemia with hemoglobin rate at 10 g/dL, serum creatinine was 1.3 mg/dL, total serum protein was 62 g/L and serum albumin was 27 g/L. The anti-nuclear antibody was positive (1/420 IU/mL), anti-double stranded deoxyribonucleic acid was positive (140 IU/mL) and IgG IgM anticardiolipin antibodies were negative. Other tests showed low C3 and C4 serum levels and negative anti-neutrophil cytoplasmic- antibodies. Abdominal-ultrasound revealed normal both kidneys in position, size and echotecture. Chest X-ray, electrocardiography and echocardiography were normal. A diagnosis of SLE was made as she met the American Rheumatology Association criteria for SLE. Renal biopsy was performed in order to establish the diagnosis and adequate therapy. It revealed class III lupus nephritis with granular peripheral and mesangial deposits of IgG+++, C1q ++, IgA++ and C3++ in all the glomeruli. She was started the treatment with oral prednisone 60 mg daily and mycophenolate mofetil (MMF) 2 g per day later increased to 3 g per day.
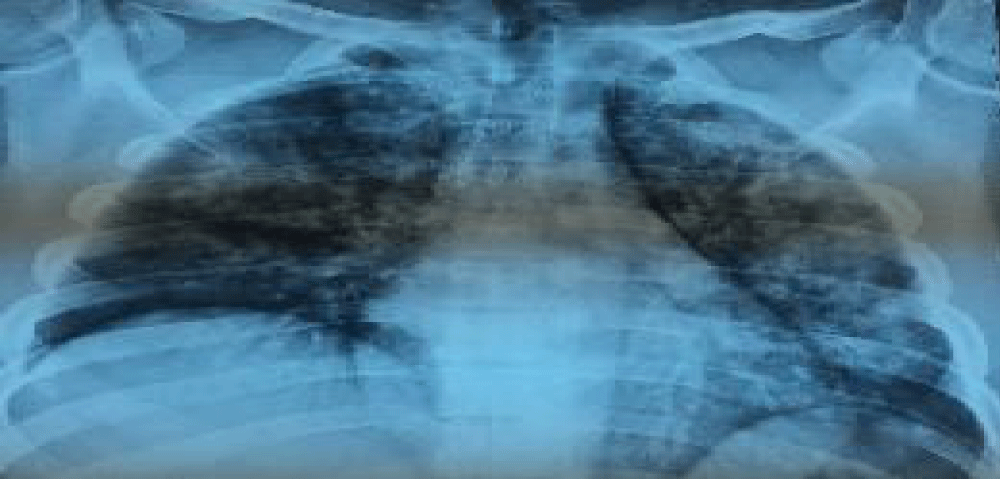
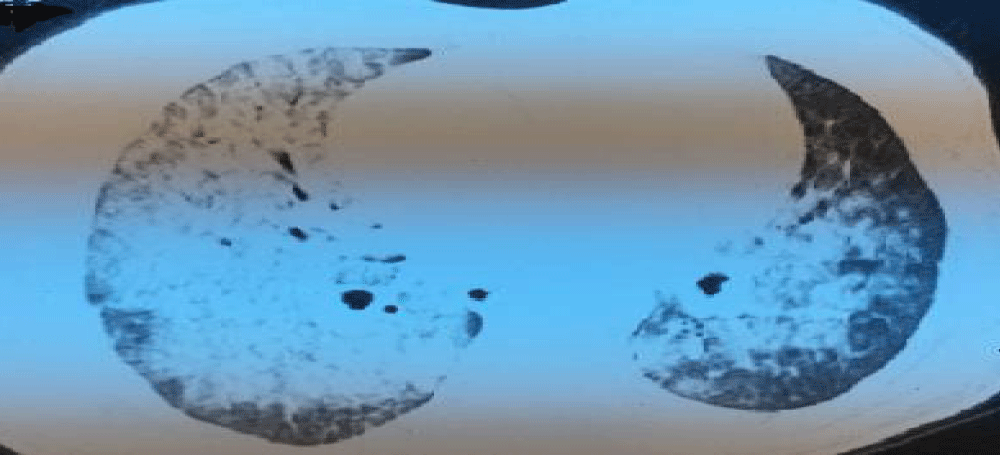
After 12 days from the initial presentation and 8 days after starting her induction-treatment, she presented with hemoptysis and rapidly progressive dyspnea. Clinically she was tachypneic and hypoxemic with room air oxygen saturation =85%. She had a pulse rate 114 beats/min a blood pressure 130/70 mmHg and a temperature of 38.3°. Her hemoglobin dropped to 6.4 g/l, the chest auscultation showed bilateral fine crackles and the heart showed normal auscultatory findings. Chest X-ray showed bilateral pulmonary infiltrates with normal cardiac silhouette (Figure 1). Computed tomography of the thorax revealed bilateral diffuse alveolar opacities in a central and perihilar distribution (Figure 2). Bronchoscopy revealed diffuse pulmonary bleeding; specimens for bronchoalveolar lavage, acid fast bacilli stain, and cultures and cytology were obtained.
Figure 1: Chest X-ray showed bilateral pulmonary infiltrates with normal cardiac silhouette.
Figure 2: Computed tomography of the thorax revealed bilateral diffuse alveolar opacities in a central and perihilar distribution.
Blood transfusions and empiric antibiotic therapy were initiated pending the microbiological results of the bronchoalveolar lavage fluid. However, the condition continued to deteriorate and she was admitted to the intensive care unit, which necessitated orotracheal intubation and mechanical ventilation.
A diagnosis of SLE with DAH was made. MMF was stopped and she was started a treatment with intravenously (IV) cyclophosphamide 1 g infusion, and IV pulse methyl-prednisolone 1 g daily for 3 days followed by 60 mg of prednisone per os daily which was tapered down gradually.
She responded well to this regimen with improvement in signs and symptoms and she was extubated on hospital day 20 and discharged on day 26. She responded well to this regimen showing improvement in respiratory symptoms allowing her extubation after 8 days of mechanical ventilation (hospital day 20). After 6 more days, the patient has been discharged. Cultures from broncho-alveolar lavage remained negative, so antibiotics were stopped. Five more monthly IV cyclophosphamide doses were programmed.
All along a period of 11 months, no recurrent bleeding has occurred and a complete remission was obtained under maintenance doses of prednisone with azathioprine.
In the current case, the diagnosis of SLE was based on the both clinical manifestation and investigation according to American Rheumatology Association criteria for SLE. Herein, DAH occurred despite ongoing induction treatment with oral corticosteroids and oral MMF for lupus nephritis. A clinical syndrome characterized by bleeding into alveolar spaces due to disruption of the alveolar–capillary basement membrane [9]. Furthermore, DAH represents a life-threatening cause of acute respiratory failure among patients with lupus and it’s considered a rare fatal complication with reported frequency ranging from 1% to 5.4% of lupus cohorts, accounting for 1.5% – 3.7% of hospital admissions due to SLE [6,7,10]. In the literature, most of the case series reported that DAH occurs usually early in the course of disease, the duration of SLE at the time of DAH ranges from 6 months to 14.1 years [6,7,10,11]. Moreover, an increased risk of occurring DAH has been described with a nephrotic syndrome, active lupus proliferative, high titers of anti-double stranded deoxyribonucleic acid and low level of hypocomplementemia [1,4,7]. In our case, DAH occurred only a few days after the initial presentation of SLE despite ongoing treatment with MMF and corticosteroids. The principal risk factor was the presence of class III lupus nephritis.
Clinical Presentation of DAH is typically acute, and patients usually show severe respiratory symptoms such as dyspnea, hypoxemia, hemoptysis and cough. Hemoptysis is a common, however it’s not an invariable manifestation of the disorder [1]. Chest X-ray may show bilateral lung infiltrates, while chest computed tomography generally shows diffuse bilateral often widespread air space, alveolar, or consolidation opacities that are non-specific [12]. Clues to the diagnosis of DAH typically include hemoptysis, a drop of hemoglobin or hematocrit and a bloody broncho-alveolar lavage fluid [1]. In our case, the presence of hemoptysis, hypoxemia, and hemoglobin drop, the chest X-ray and computed tomography findings and a bloody broncho-alveolar lavage fluid with a negative culture confirmed the diagnosis of DAH, whereas active lupus proliferative nephritis was an associated risk factor.
Since DAH is a rare complication of SLE, a prospective-controlled-studies are lacking, so guidelines for management of DAH have not been reported. In our case, the use of combination of pulse methylprednisolone and cyclophosphamide was associated with a good response. In study by Zamora, et al. plasmapheresis use in SLE with DAH has not been proven to be as effective as in other autoimmune causes (Good pasture’s syndrome, some vasculitis-related pulmonary bleeding), that’s why it may be beneficial only when there is no response to high-dose of corticosteroids and cyclophosphamide [13-15]. Others promising options such as rituximab and pulmonary administration recombinant-activated human factor VIIa can be used and have been effective in stopping bleeding [16,17].
Our conclusion is that DAH is a rare complication of SLE and carries a high mortality rate. It’s can occur despite ongoing induction treatment with corticosteroids and immunosuppressive therapy. Active lupus nephritis is a major risk factor, hence early recognition and aggressive therapy needs to be started promptly.
- Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus: clinical presentation, histology, survival, and outcome. Medicine. 1997; 76: 192-202. PubMed: https://pubmed.ncbi.nlm.nih.gov/9193454/
- Hunninghake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis.1979; 119: 471-503.
- Emlen W. Systemic lupus erythematosus and mixed connective tissue disease. Immunol Allergy Clin North America. 1993; 13: 291–311.
- Santiago-Casas Y, Vila LM. Pulmonary hemorrhage in patients with systemic lupus erythematosus. Curr Respir Med Rev. 2009; 5: 49-54.
- Kamen DL, Strange C. Pulmonary manifestations of systemic lupus erythematosus. Clin Chest Med. 2010; 31:479-488. PubMed: https://pubmed.ncbi.nlm.nih.gov/20692540/
- Koh WH, Thumboo J, Boey ML. Pulmonary haemorrhage in oriental patients with systemic lupus erythematosus. Lupus 1997; 6: 713–716.
- Badsha H, Teh CL, Kong KO, Lian TY, Chng HH. Pulmonary hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 2004; 33: 414–421. PubMed: https://pubmed.ncbi.nlm.nih.gov/15190526/
- Olser W. On the visceral manifestations of the erythema group of skin diseases. Am J Med Sci. 2009; 338: 396–408. PubMed: https://pubmed.ncbi.nlm.nih.gov/19938295/
- Alabed LB. Treatment of Diffuse Alveolar Hemorrhage in Systemic Lupus Erythematosus Patient with Local Pulmonary Administration of Factor VIIa (rFVIIa) A Case Report. Medicine. 2014; 93: 14. PubMed: https://pubmed.ncbi.nlm.nih.gov/25255019/
- Liu MF, Lee JH, Weng TH, Lee YY. Clinical experience of 13 cases with severe pulmonary hemorrhage in systemic lupus erythematosus with active nephritis. Scand J Rheumatol. 1998; 27: 291–295.
- Martınez-Martınez MU, Abud-Mendoza C. Diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Clinical manifestations, treatment, and prognosis. Reumatol Clin. 2014; 10: 248–253.
- Marten K, Schnyder P, Schirg E, Prokop M, Rummeny EJ, et al. Pattern-based differential diagnosis in pulmonary vasculitis using volumetric CT. Am J Roentgenol 2005; 184: 720–733.
- Hoshi KI, Matsuda M, Ishikawa M, Mitsuhashi S, Gono T, et al. Successful treatment of fulminant pulmonary hemorrhage associated with systemic lupus erythematosus. Clin Rheumatol. 2004; 23: 252-255.
- Huang DF,Tsai TS, Wang SR. Recovery of both acute massive pulmonary hemorrhage and acute renal failure in a systemic lupus erythematosus patient with lupus anticoagulant by the combined therapy of plasmapheresis plus cyclophosphamide. Transfusion Science. 1994; 15: 283-288.
- Claridge S, Das P, Dorling A, Robson M. Plasmapheresis as rescue therapy for systemic lupus erthyematosus associated diffuse alveolar haemorrhage. BMJ Case Rep. 2011; 2011. PubMed: https://pubmed.ncbi.nlm.nih.gov/22698899/
- Na JO, Chang SH, Seo KH, Sung Choi J, Sung Lee H. et al. Successful Early Rituximab Treatment in a Case of Systemic Lupus Erythematosus with Potentially Fatal Diffuse Alveolar Hemorrhage. Respiration. 2015; 89: 62–65.
- Estella A, Jareno A, Perez-Bello L. Intrapulmonary administration of recombinant activated factor VII in diffuse alveolar haemorrhage: a report of two case. Cases J. 2008 12; 1: 150. PubMed: https://pubmed.ncbi.nlm.nih.gov/18789132/