More Information
Submitted: 30 July 2020 | Approved: 20 August 2020 | Published: 21 August 2020
How to cite this article: Rico-Fontalvo J, Daza-Arnedo R, Cardona-Blanco MX, Leal-Martínez V, Abuabara E, et al. SGLT2 Inhibitors and nephroprotection in diabetic kidney disease: From mechanisms of action to the latest evidence in the literature. J Clini Nephrol. 2020; 4: 044-055.
DOI: 10.29328/journal.jcn.1001058
Copyright License: © 2020 Rico-Fontalvo J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Kidney failure; Chronic; Sodium-glucose transporter 2; Diabetic nephropathies; Diabetes mellitus; Proteinuria (MeSH)
SGLT2 Inhibitors and nephroprotection in diabetic kidney disease: From mechanisms of action to the latest evidence in the literature
Jorge Rico-Fontalvo1(ORCID: 0000-0002-2852-1241), Rodrigo Daza-Arnedo2(ORCID: 0000-0002-6295-4972), Maria Ximena Cardona-Blanco3(ORCID: 0000-0003-3918-7645), Victor Leal-Martínez4(ORCID: 0000-0003-1228-148X), Emilio Abuabara-Franco5(ORCID: 0000-0001-5098-6574), Nehomar Pajaro-Galvis6*(ORCID: 0000-0001-6682-1918), Jose Cabrales7(ORCID: 0000-0002-2584-6999), José Correa8(ORCID: 0000-0002-9736-4913), Manuel Cueto9(ORCID: 0000-0003-3915-7294), Amable Duran10(ORCID: 0000-0003-1895-1907), Alejandro Castellanos11(ORCID: 0000-0002-8822-6770), Javier Enamorado12(ORCID: 0000-0002-1828-7674), José Bohórquez13(ORCID: 0000-0002-9064-1068), Isabella Uparella14(ORCID: 0000-0003-2940-5362), Julio Zuñiga15(ORCID: 0000-0002-8018-6057), Abraham Chagui16(ORCID: 0000-0002-9818-2740), Alfonso Ramos17(ORCID: 0000-0003-2704-898X) and Luis Lara18(ORCID: 0000-0001-9186-1866)18
1Nephrologist, Nephrodiabetes Committee, Colombian Nephrology Association, Medellín, CO
2Nephrologist, Nephrodiabetes Committee, Colombian Nephrology Association, Cartagena, CO
3Nephrologist, Colombian Nephrology Association, Medellín, Colombia
4Internist, New Bocagrande Hospital, Cartagena, CO
5Internist, Concepcion clinic, Cartagena, CO
6Resident Physician, Department of Internal Medicine, University of Sinu, Cartagena, CO
7Resident Phyisician, Department of Internal Medicine, St. Elizabeth’s Medical Center/Tufts University School of Medicine, Boston, MA, USA
8Internist, Critical Medicine and Intensive Care Fellow, University of Cartagena, Cartagena, CO
9Nephrologist, Iberoamerican Clinic, Barranquilla, CO
10Nephrologist, Colombian Nephrology Association, CO
11Internist, Endocrinologist, San Jeronimo Hospital, Monteria, CO
12Physician, Jose bauza frau office, Santiago de Chile
13Medical Student, University of Sinu, Cartagena, CO
14Medical Student, University of Sinu, Cartagena, CO
15Physician, Rafael Núñez University, CO
16Physician, University of Sinu, CO
17Physician, Gestion Salud Clinic, CO
18Physician, Estrios Clinic, CO
*Address for Correspondence: Nehomar Pajaro-Galvis, Third year Resident Physician, Department of Internal Medicine, University of Sinu, Colombia, Tel: 57+ 3008233570; Email: [email protected]
Type 2 Diabetes Mellitus constitutes a major problem in public health worldwide. The disease poses a high risk of severe microvascular and macrovascular complications. Diabetic kidney disease is the most common cause of end-stage chronic kidney disease and contributes to the increasing morbidity and mortality associated to diabetes. Sodium-glucose contransporter-2 inhibitors (SGLT2 inhibitors) are the latest oral diabetic medications, which exhibit a great nephroprotective potential, not only by improving glycemic control, but also by glucose-independent mechanisms, such as decreasing blood pressure and other direct renal effects. We conduct a literature review based on the most recent scientific evidence with the goal to elucidate the postulated mechanisms of action of SGLT2 inhibitors in diabetic kidney disease, which are the base of the beneficial clinical effects that are seen in the condition.
Type 2 Diabetes Mellitus (T2D) constitutes a major problem in public health worldwide [1]. Its major impact is in the vasculature; it poses a risk of microvascular and macrovascular complications as a consequence of the constant hyperglycemic state [2]. Approximately half of the patients with T2D develop diabetic kidney disease at some point in their evolution. Diabetic kidney disease (DKD) is considered a major microvascular complication as it leads to ends stage kidney disease (ESKD), contributing to increased mortality [1-4]. Patients with DKD and ESKD can have a mortality risk that is 3 to 12 times higher than patients without these complications [5]. There is evidence suggesting that poor glycemic control can explain the pathogeny of the structural damage of the nephrons that occurs primarily in the mesangium, later on progressing to diffuse damage, capable of progressing to ESKD [6,7].
Nowadays, we lack the development of a medication targeted specifically towards DKD, with the mainstay of treatment being glycemic control, blood pressure and lipid management. However, control of these factors is not enough to attenuate the incidence and progression of DKD [8]. In this setting, SGLT2 inhibitors are a new class of oral hypoglycemic drugs suggested to exert nephroprotective effects through glucose-dependent and glucose-independent mechanisms [8]. The nephroprotective effect of SGLT2 inhibitors has been demonstrated in randomized clinical trials (RCT) in patients with T2D and high cardiovascular risk, which support their use. There favorable effects of SGLT2 inhibitors are explained by mechanisms that act upon the pathogenic pathways of DKD, including glomerular hyperfiltration, inflammation and oxidative stress [8,9]. Currently, there are 3 drugs that represent SGLT2 inhibitors: empaglifozin, canaglifozin and dapagliflozin.
History of Type 2 diabetes mellitus
Type 2 Diabetes Mellitus is a disease that has had marked the history of humankind given the impact it has on the quality of life on the patients that suffer from it [11]. T2D is the most common type of diabetes and its pathophysiololgic mechanisms have allowed the use of oral hypoglycemic agents like biguanides, sulfonylureas, dipeptidyl-peptidase 4 (DPP-4) inhibitors, among others. However, the previously mentioned drugs have side effects that sometimes may lead to difficulty in the management of the disease. Hence, there was a call for the development of new agents, capable of exhibiting less hostility and better efficacy through new mechanisms for the control of this disease [12,13].
In 1835, a substance was isolated from the cortex of the apple called phlorizin, a natural O-glucoside. Given its similar properties to Cinchona extracts and weeping willow, it was proposed as a reasonable candidate to treat fever, some infectious diseases and even malaria. Approximately 50 years later, its glucosuric capacity at high doses was discovered [11,14,15]. It was also suggested that phlorizine induced diabetes in canine models, given that it caused similar symptoms with its chronic administration (glucosuria, polyuria and weight loss) [15].
In 1930, the kinetics of glucose reabsorption at the level of the kidney were demonstrated for the first time [3].
Studies regarding phlorizine continued in the next few decades focusing on renal physiology, and 40 years later it was discovered that active glucose transport responsible for the reabsorption of glucose was located in the brush border membrane of the proximal tubule, site where phlorizine had its action. It was then when the kidney started to be conceptualized as an important organ in the metabolism of glucose, contemplating it as a possible target in glucose lowering therapy [13-15].
Between the years 1980 and 1990, sodium-glucose cotransporters-1 (SGLT1) and sodium-glucose cotransporters-2 (SGLT2) inhibitors were identified, as well as the benefits of phlorizine in the diabetic population primarily in animal models. In diabetic rats with to 90% pancreatectomy, it was discovered that phlorizine normalized fasting and postprandial glucose levels, with additional benefits in insulin resistance [14,15]. In contrast to the previously mentioned, phlorizine was not thought of a candidate for glucose lowering therapy, given a great number of limitations. The first critical limitation is that it’s not a selective inhibitor, acting on SGLT1 and SGLT2, leading to gastrointestinal side effects such as diarrhea and dehydration. Other limiting factors include the fact that it’s metabolically unstable given the cleavage of glucosidase in the gastrointestinal tract, hindering its oral administration, among others.
Given the previously mentioned limiting factors, pharmaceutical companies promoted large investigations to create phlorizine analogs, with the aim of improved oral bioavailability, better metabolic stability and selectivity. Serglifozin, remoglifozin, AVE2268 and T-1095 were created, which showed a dose-dependent decrease in the reabsorption of glucose at the level of the kidney, as well as suppression of hyperglycemia; additionally, they exhibited increased selectivity for SGLT2 in comparison to phlorizine. Nonetheless, the pharmaceutical companies and the scientific community and the pharmaceutical companies continued the investigations given that these drugs had poor pharmacokinetic stability and their selectivity was incomplete, leading to the discovery of C-Aryl glucosidase SGLT2 inhibitors in the year 2000 [11,13].
In opposition to O-glucosides and its analogues, C-glucosides were resistant to β-glucosidases, increasing their half-life ad their selectivity SGLT2 over SGLT1 (depending of the agent utilized) [12,16]. In the year 2008, Meng, et al. discovered Dapaglifozin, which was admired as a potential tool for the treatment of type 2 diabetes [15]. Multiple studies have shown a favorable response with the use of Dapaglifozin, regulating fasting and post-prandial serum glucose [16]. Dapaglifozin was approved for use in the market for the first time in Europe in 2012; the Food and Drug Administration (FDA) approved the medication for the treatment of T2D in January 2014 [17].
In the years 2010 and 2012, Canaglifozin [18] and Empaglifozin [19] were synthesized, respectively. Empaglifozin is the agent with the highest selectivity of all the previously mentioned. These drugs were approved in the following years by the DFA and the European Medicines Agency (EMA). In that sense, the development of C-glucosides continued, leading to the creation of medications such as sotaglifozin, serglifozin, ipraglifozin, tofoglifozin and luseoglifozin; it is known that some of these medications are inhibitors of both SGLT1 and SGLT2. The investigations continue to evaluate the possibility of a superior benefit with mixed inhibitors, however, evidence is required, and it depends on the individual characteristics of each patient [16].
The role of the kidney in glucose homeostasis
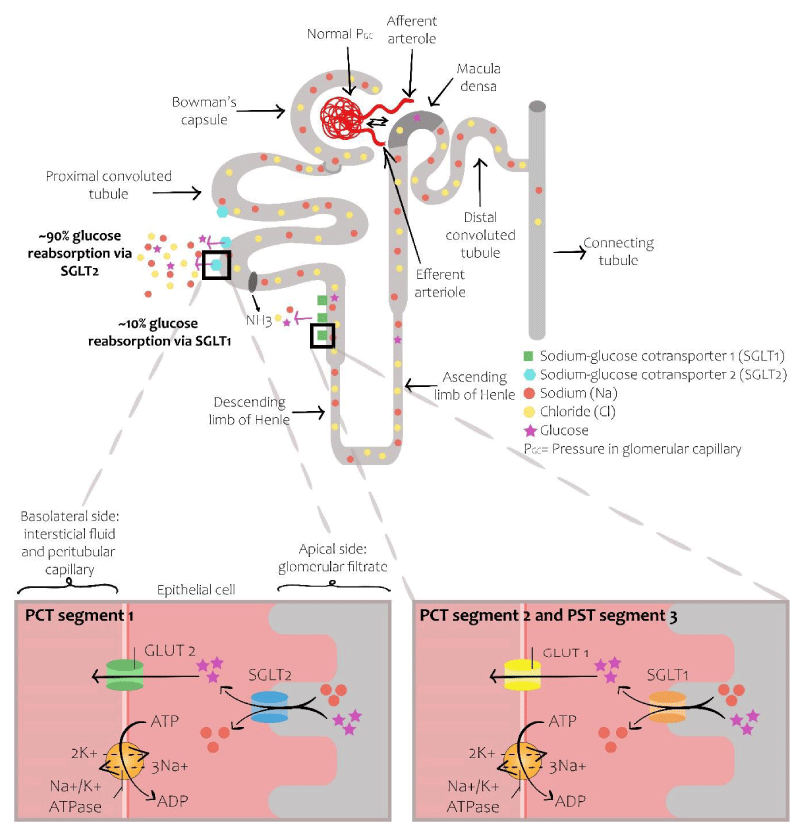
The kidney plays a crucial role in glucose homeostasis. In euglycemic or slightly hyperglycemic conditions, the kidney is capable of reabsorbing almost all the glucose in the glomerular filtrate. Glucose reabsorption occurs in the proximal tubules via the sodium symporters expressing themselves in the proximal tubule. Of these, SGLT 1 and SGLT2 are the main ones [3]. Both transporters complement themselves and permit almost complete reabsorption of the filtered glucose. SGLT2 are low affinity and high capacity glucose co-transporters localized in the lumen of proximal tubular cells, exerting their action specifically in the S1 segment of the proximal tubule; they conduct glucose and sodium inside the cells and are responsible for the reabsorption of 80% to 90% of the filtered glucose. The remaining 10% to 20% is reabsorbed by SGLT1, high affinity and low capacity glucose co-transporters, expressed in the distal part of the proximal tubule; they exert their action specifically in the S2 and S3 segments of the proximal tubule [3,5,10,20,21]. The entry of sodium inside the cells through SGLT1 and 2 is driven by a concentration gradient of sodium generated by the sodium-potassium ATPase (Na-K ATPase) pump, located in the basolateral membrane. The exit of sodium through the pump decreases intracellular sodium, leading to entry of tubular sodium inside the cell. After reabsorption, glucose moves passively inside the interstitial space utilizing the glucose transporters GLUT1 and GLUT2, expressed on the basolateral membrane of the proximal tubular cells [3,10] (Figure 1).
Figure 1: Normal nephron. The reabsorption of glucose is produced through the co-transporters SGL T1 and SGLT2, expressed in the apical membrane of the proximal tubule. SGLT2 reabsorbs approximately around 80% to 90% of the filtered glucose. The resting 10% to 20% is reabsorbed by SGLT1, a high affinity and low capacity transporter that is expressed in the apical membrane of the epithelium in the proximal descending tubule.
In human beings, a serum glucose of 180 mg/dl is considered the threshold to overwhelm the capacity of renal reabsorption [3.10], higher values will cause glucosuria. However, the threshold can vary in between 100 -240 mg/dl. Diabetes increases the glucosuric threshold to 200-240 mg/dl, exacerbating hyperglycemia [3]. The exact mechanism behind this response is not well known; however, multiple studies show that the increase in the expression and activity of SGLT2 is the cause of a higher reabsorption of glucose by the diabetic kidney [3,20,22].
Type 2 diabetes and renal compromise
Despite the fact that diabetic kidney disease depends on the interaction of multiple factors and processes, the main pathways implicated in its pathophysiology are: Glomerular hyperfiltration (imposed by metabolic alterations that include hyperglycemia and hyperaminoacidemia), inflammatory processes and an increase in oxidative stress [3,8]. These pathophysiological pathways culminate by generating structural and functional alterations, manifested typically with albuminuria, a decrease in the glomerular filtration rate and hypertension [10].
Type 2 diabetes induces glomerular capillary hypertension and hyperfiltration. The pathophysiological mechanisms of the latter are not completely understood; however, the hemodynamic hypothesis and the tubular hypothesis are the most likely explanations [9,22]. The hemodynamic hypothesis is based on changes in the afferent and efferent arteriolar tone, resulting in hyperfiltration, mainly due to the activation of the renin-angiotensin-aldosterone system (RAAS) [20,22]. This means that in a hyperglycemic state, the capacity of reabsorption of tubular glucose increase: hence, sodium reabsorption increases. Given this, a lower sodium concentration reaches the macular densa, similarly to renal hypoperfusion, and through tubular glomerular feedback, the RAAS system is activated producing afferent arteriolar vasodilatation and efferent arteriolar vasoconstriction, increasing GFR [9,10,22].
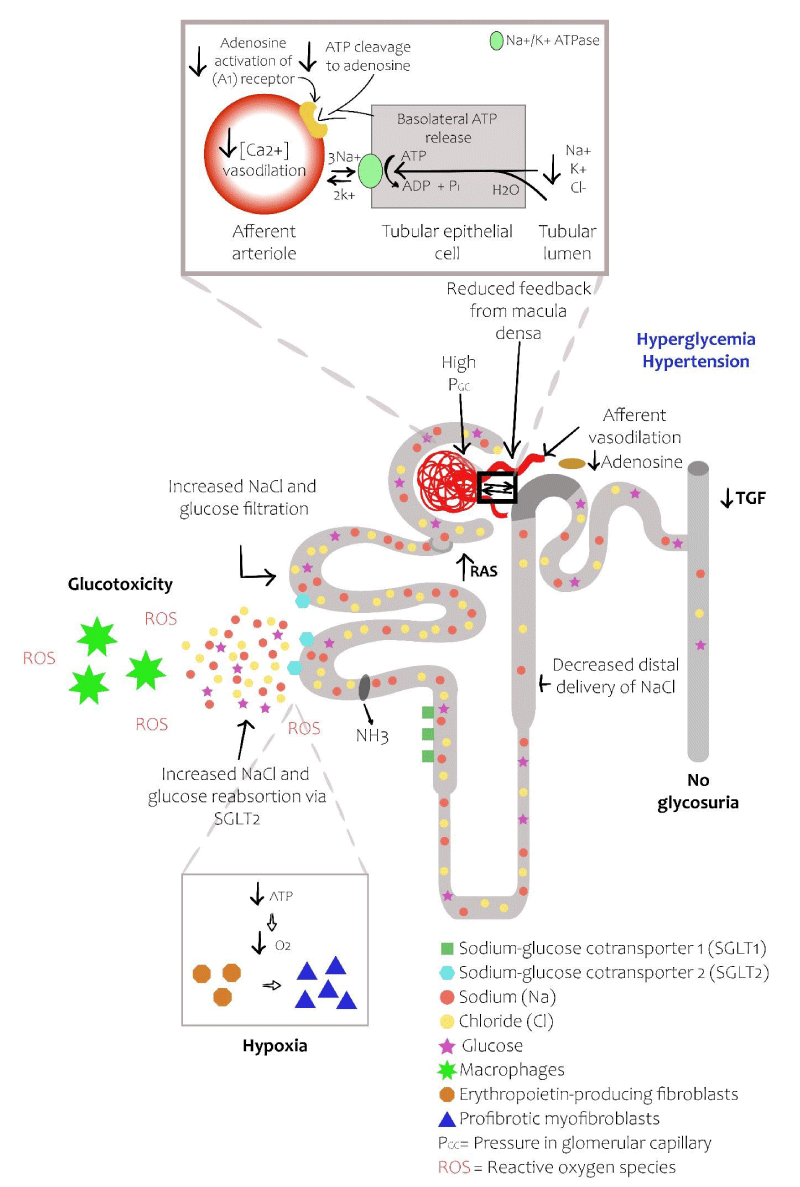
The tubular hypothesis is based on the fact that hyperglycemia leads to an increase in the filtered glucose in the proximal tubule in the hyperglycemic state; augmenting the expression and activity of SGLT2, increasing the reabsorption of glucose and sodium, leading to worsen glucose control and increasing blood pressure. Additionally, the reabsorption of proximal sodium produces a decrease in the availability of sodium in the distal tubules and the decrease in the sodium reaching the macular densa, leading to a reduction in the hydro-lysis of ATP and the production of adenosine. Adenosine is a potent vasoconstrictor, and its reduction, such as in the hyperglycemic state, causes vasodilatation of the afferent arteriole, increasing intra-glomerular pressure and filtration [3,5,20,22]. The previously mentioned changes at the glomerular level produce inflammation and oxidative stress, with an increase in the production of pro inflammatory cytokines and reactive oxygen species (ROS). The latter leads to the production of excessive extracellular matrix, generating fibrosis which leads to interstitial tubular damage and contributes to podocyte damage via the endothelial-podocyte cross-talk. Finally, increasing oxygen consumption in the renal cortex can contribute to renal fibrosis by inducing hypoxia and trans-differentiation of EPO producing fibroblasts in the pro-fibrotic myofibroblasts (Myo-Fb) [9,10,24] (Figure 2).
Figure 2: Diabetic nephron. The increase in reabsorption of glucose by SGLT2 in the proximal convoluted tubule decreases the delivery of solutes to the macular density. The resulting decrease in ATP release from the basolateral membrane of the epithelial tubular cells reduces the production of adenosine produces vasodilatation of the afferent arteriole, leading to hyperfiltration and an increase in the glomerular capillary pressure. The increase infiltrate glucose produces inflammation and oxidative stress. Finally, the increase in oxygen consumption of the renal cortex can contribute to renal fibrosis by inducing hypoxia and differentiation of EPO producing fibroblasts in pro-fibrotic myofibroblasts (adapted from references 3 and 5).
Effects of SGLT2 inhibitors in the kidney
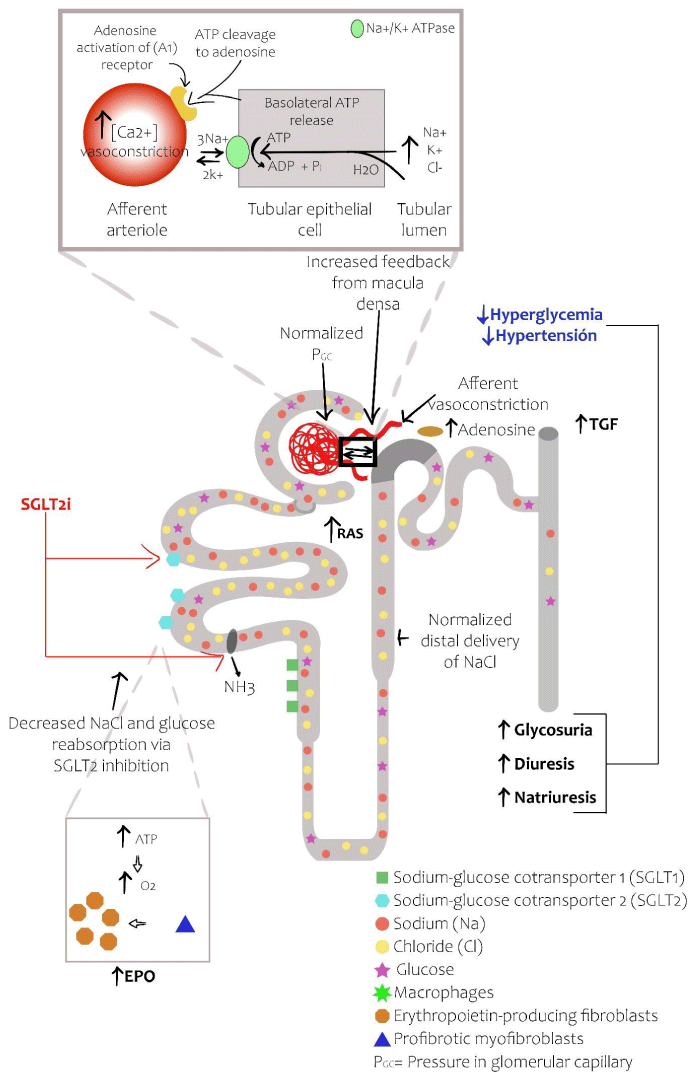
Although a decrease in the serum glucose is fundamental for the prevention of diabetic kidney disease, it is also likely that there are direct and independent nephroprotective effects of blood glucose. There is increasing knowledge about the mechanisms involved in the nephroprotective effects of SGLT2 inhibitors [3]. As previously mentioned, there is an increase in the expression capacity of glucose transport by SGLT2 in type 2 diabetes [20]. Hence, inhibition of SGLT2 leads to a decreased reabsorption of glucose in the proximal tubule, increasing the availability of sodium, allowing it to reach the distal portions of the nephron, especially the macula densa. This restores the glomerular-tubular feedback. The latter results in an increase of sodium delivery to the macula densa, leading to a local liberation of adenosine and as a consequence, intracellular calcium, generating vasoconstriction of the afferent arteriole [3,10,20,25]. Eventually, there is a reduction in glomerular hyperfiltration and in intraglomerular hypertension, attenuating albuminuria, which are the primary pathophysiological mechanisms of diabetic kidney disease, explaining the benefits beyond reducing serum glucose [10]. It is worth mentioning that the mechanism of SGLT2 inhibitors is different from the ones seen in RAAS blockers, given that these agents also decrease intraglomerular pressure by causing vasodilation of the efferent arteriole [26,27]. RAAS inhibitors act by either inhibiting the angiotensin-converting enzyme (ACE) or by blocking the angiotensin II type 1 receptor (ACE inhibitors and angiotensin receptor blockers, respectively), thereby reducing the activity of angiotensin II leading to vasodilatation of the efferent arteriole and a reduction of the intraglomerular pressure [47]. On the other hand, as mentioned previously, SGLT2 inhibitors cause an indirect vasoconstriction of the afferent arteriole, which in turn also reduces intraglomerular pressure. By these mechanisms, SGLT2 inhibitors and RAAS system inhibitors help to slow down the progression of DKD.
Additional to the beneficial action in glomerular hemodynamics, other effects seem to contribute positively in regards to diabetic kidney disease; however, in less proportion. SGLT2 inhibitors are also capable of reducing renal energy requirements, reducing renal hypoxia. Similarly, they pose an anti-inflammatory, antioxidant and anti-fibrotic action, given a reduction in glycogen end products, the expression of pro inflammatory molecules and reactive oxygen species [10,21] (Figure 3). SGLT2 inhibitors have demonstrated anti-inflammatory effects by reducing serum levels of IL-6 and leptin, decreasing C reactive protein (CRP) levels, increasing adiponectin and inhibition the IL-1β secretion by macrophages via the ROS-NLRP3-caspase-1 pathway [25]. Some SGLT2 inhibitors such as luseoglifozin have shown to decrease the expression of hypoxia inducible factor 1α (HIF 1α), which plays an important role in hypoxia induced tubulointerstitial fibrosis. In addition to the previously mentioned, they also inhibit the expression of HIF 1α target genes PAI-1, VEGF, GLUT1, HK2 and PKM [48]. Canaglifozin has demonstrated to modulate key nutrient-sensing pathways via the activation of 5´ AMP-activated protein kinase (AMPK) and by inhibiting the mechanistic target of rapamycin (mTOR), which is independent of glucagon, reducing adiposity and improving glucose tolerance in the setting of reduced serum insulin [49].
Figure 3: Effects of SGLT2 inhibitors in the diabetic nephron. SGLT2 inhibitors restore the delivery of solutes to the macular densa with the subsequent activation of adenosine and the reversal of the vasodilatation of the afferent arteriole. In patients treated with SGLT2 inhibitors, glucosuria, natriuresis and osmotic diuresis reduce the levels of glucose in the blood and lower blood pressure. By inhibiting the reabsorption of sodium at the level of SGLT2 as well as the sodium-hydrogen exchanger, the SGLT2 inhibitors restore the GFR with a decrease in the glomerular capillary pressure. The reduced reabsorption of glucose decreases local glucose toxicity. The improvement of the hypoxia in the renal cortex allows for differentiation of myofibroblasts in the EPO producing cells, reducing renal fibrosis and increasing EPO production [3,5].
Effects of SGLT2 inhibitors on DKD risk factors
It is known that glycemic control decreases the risk of diabetic kidney disease, particularly if implemented early in the course of the disease. Recent meta-analysis of clinical trials in which SGLT2 inhibitors were used as monotherapy or as complementary treatment demonstrated a reduction in hemoglobin A1c (HBA1c) of 0.6 -0.7% [9]. However, given the intrinsic mechanism of action, the glucose lowering effect of SGLT2 inhibitors is reduced in patients with low estimated GFR (eGFR). Finally, the increasing oxygen consumption of the renal cortex can contribute to fibrosis of the kidney by inducing hypoxia and differentiation of erythropoietin producing trans-fibroblasts in pro-fibrotic myofibroblasts (MyoFb) [3].
It has been shown that the loss of corporal fat per se is capable of decreasing the albuminuria in the glomerular hyperfiltration; hence, the effect of weight loss of SGLT2 inhibitors can protect indirectly the diabetic kidney. The glucosuria induced by SGLT2 inhibitors decreases body weight through caloric losses [3,5]. In patients with normal renal function, SGLT2 inhibitors lead to weight loss of 60 to 80 g of glucose (240 -320 cal) per month. However, weight loss stabilizes after 6 months of treatment after achieving a great loss of 5 to 7 pounds (2.3 to 3.2 kg) [3].
SGLT2 inhibitors also pose an antihypertensive effect, which is very relevant in the context of diabetic kidney disease, given the control of blood pressure is a challenge in patients with chronic kidney disease and hypertension plays a key role in albuminuria and in the progression of chronic kidney disease. With the use of SGLT2 inhibitors the blood pressure lowering activity is reached without compensatory increase in the heart rate [14]. SGLT2 inhibitors are capable of decreasing systolic and diastolic blood pressure approximately 4 to 5 mmHg and 2 mmHg, respectively [8,14]. Natriuresis and osmotic diuresis have been proposed as adjacent mechanisms; the inhibition of sodium reabsorption at the level of SGLT2 leads to inhibition of other transporters of sodium like an NHE3, leading to a sustained decrease in plasma volume, with a subsequent reduction in sympathetic tone and vascular rigidity, resulting in a higher elasticity [3,5,24,25].
Similarly, SGLT2 inhibitors have a uricosuric effect. Uric acid, additional to being a risk marker and predictor of renal insufficiency, can also have a pathogenic role in microvascular damage in general and in diabetic kidney disease, specifically. SGLT2 inhibitors decrease the uric acid through the alteration of its tubular transport. The two best described transporters for uric acid are URAT1 and GLUT9, the inhibition of tubular reabsorption through these transporters by glucosuria is considered the main mechanism, hence, these agents increase the excretion of uric acid in a dose-dependent manner. Also, as insulin induces the expression of URAT1, SGLT2 inhibitors can also improve uricosuria by improving glucose control and suppression of insulin [5,25,28,29].
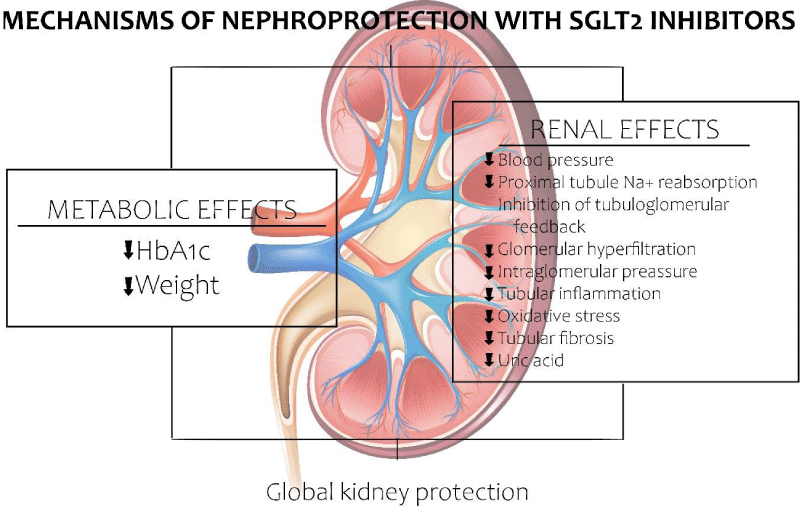
As mentioned previously, in hyperglycemic conditions, a great amount of oxygen is required in the proximal tubule given the excessive reabsorption of glucose, leading eventually to tubulointerstitial hypoxia. The treatment with SGLT2 inhibitors decreases the reabsorption of glucose which decreases local glucose toxicity. In other words, the use of SGLT2 inhibitors improves hypoxia at the level of the renal cortex, allowing differentiation of myofibroblasts to EPO producing cells, which increases the production of EPO and limits renal fibrosis. Given the latter, patients with diabetic kidney disease have almost doubled the probabilities of developing anemia in comparison to patients with chronic kidney disease not related to diabetes and similar eGFR’s. Additionally, treatment with SGLT2 inhibitors increases hematocrit values as well as EPO synthesis [28]. Recent clinical and basic science studies have demonstrated that EPO has direct nephroprotective effects additional to correcting the anemia. For example, it has been demonstrated that EPO prevents nephrotic syndrome induced by podocyte damage. Similarly, Eren, et al. discovered that EPO inhibits inflammatory markers and oxidative stress, attenuating albuminuria and reducing tubular damage, inflammation and interstitial fibrosis [30]. A clinical study demonstrated that EPO stimulating agents reduced the decrease in EGFR in patients with chronic kidney disease [31]. These findings indicate that EPO also direct nephroprotective effects, which are enhanced with the use of SGLT2 inhibitors (Figure 4).
Figure 4: Mechanisms of Nephroprotection with the use of SGLT2 inhibitors.
Relevant clinical studies and diabetic kidney disease and SGLT2 inhibitors
Type 2 diabetes complications, especially diabetic kidney disease, still pose a great challenge for clinicians. For its control it has been proposed the use of our RAAS system inhibitors, with some clinical benefit; however, its limitations make them insufficient. Even with aggressive management of double blockade and serum glucose control, it is still insufficient, reflected in the number of patients with end-stage kidney disease and in renal replacement therapy. Given this, the scientific community has continued its investigations, through clinical trials and performing analysis of these, contemplating SGLT2 inhibitors as a tentative therapy for a more assertive management, given the benefits that are not just limited to glycemic control [5,22,28].
Current evidence has shown that SGLT2 inhibitors not only decrease serum glucose, but also have vascular and renal protective effects (5). These benefits have been reported in several clinical studies which include: EMPA-REG OUTCOME (Empaglofizin), CANVAS and CREDENCE (Canaglifozin), and DECLARE- TIMI 58 (dapaglifozin). They will be analyzed with emphasis and renal outcomes of the patients in the studies [27].
EMPA-REG OUTCOME (empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes) was a multicentric, double-blind study mainly with a cardiovascular focus, which randomly assigned 7028 patients with type 2 diabetes and established cardiovascular disease and 42 countries to either empagliflozin or placebo. The study demonstrated that empagliflozin reduces the risk of incidence and progression of diabetic kidney disease in comparison to placebo in patients with T2D and high cardiovascular risk. The study also revealed reduction in the progression of macroalbuminuria, duplicating serum creatinine in patients with EGFR less than 45 mL/min/1.73 m², as well as the need for renal replacement therapy. An initial short-term decrease in the EGFR in diabetic patients with SGLT2 inhibitors was observed. However, this decrease was corrected with the administration of long-term therapy, and from that point, the eGFR was stable, while it continued to decrease constantly in the placebo group [32].
The CANVAS (study for the cardiovascular evaluation of canagliflozin) was a multicentric, randomized clinical trial that included 10,142 patients, and had the main objective to analyze cardiovascular mortality, nonfatal myocardial infarction, and nonfatal cerebrovascular accident in patients treated with canagliflozin. The renal outcomes showed possible benefits in relation to the progression of albuminuria. The progression of albuminuria was defined in the study as an increase of more than 30% in pre-existing albuminuria or change in the state of normal albuminuria to microalbuminuria, from normal albuminuria to macroalbuminuria, or from microalbuminuria to macroalbuminuria. The study demonstrated that type 2 diabetes patients with high vascular risk treated with canagliflozin had a 40% reduction in the composite renal outcome [33].
The CREDENCE study (canagliflozin and renal outcomes and type 2 diabetes and nephropathy) is one of the most important in regards to the renal chapter given that it has the particular design to determine the nephroprotective effects of canagliflozin in the context of the standard management (RAAS blockers). The study had a sample of 4401 patients with T2D and an EGFR of 30 to 90 mL/min/1.73 m² and severely increased albuminuria (> 300 mg/g of albumin to creatinine in the urine); the medium follow-up was 2.62 years. The rate of events and the primary outcome was 30% less in the group on canagliflozin compared to the placebo group, decreasing the risk of dialysis, transplant or a sustained reduction of the eGFR in patients with type 2 diabetes and nephropathy (EGFR of 30 to 90 mL/min/1.73 m²). Canagliflozin reduced the primary results of the study, which was a composite of end-stage kidney disease, duplication of serum creatinine or death by renal causes of cardiovascular causes by 30%. These risks decreased significantly, with a number needed to treat (NNT) to prevent end-stage kidney disease, the duplication of serum creatinine or death by renal disease of 28, and 43 only for end-stage kidney disease. In summary, the CREDENCE trial demonstrated protective renal and cardiovascular effects additional to standard therapy in patients with diabetic kidney disease [34].
The DECLARE-TIMI-58 (dapagliflozin and cardiovascular outcomes and type 2 diabetes) was a multicentric, randomized trial with a sample of 17,160 patients, that evaluated the clinical results of dapagliflozin in patients with type 2 diabetes and established cardiovascular disease or high cardiovascular risk, observed approximately for 4 years. In the renal addendum, the results showed an improvement in the renal component (decrease of more than 40% in the eGFR to less than 60 mL/min/1.73 m², de novoend-stage kidney disease or death by renal cardiovascular causes) in individuals with type 2 diabetes and a high cardiovascular risk treated with dapagliflozin in comparison to those treated with placebo. In the general population, the incidence of the composite renal outcome was 4.3% in the dapagliflozin group versus 5.6% in the placebo group [35] (Table 1).
| Table 1: Summary of the main clinical studies evaluating the results of SGLT2 inhibitors and renal outcomes. | ||||||
| Study | SGLT-2 | Design | Population | Sample | Duration | Impact on Renal Outcomes (reduction of eGFR > 40%, doubling of sCr o ESKD) |
| EMPA-REG OUTCOME | Empaglifozin | Randomized, Double Blind |
T2D with Cardiovascular Disease | 7,028 | 3,1 years | 46% |
| CANVAS Program (33) |
Canaglifozin | Randomized, Double Blind | T2D with High Cardiovascular Risk | 10,142 | 188 weeks | 40% |
| DECLARE-TIMI 58 (35) |
Dapaglifozin | Randomized, Double Blind |
T2D with Risk Factor sor Cardiovascular Dosease | 17,160 | 4,2 years | 47% |
| CREDENCE (34) | Canaglifozin | Randomized, Double Blind |
T2D GFR < 90 ml/min/1,73m2 Albumin to creatinine ratio: 300– 5,000 mg/g |
4,401 | 2,6 years | 30% |
| VERTIS | Ertugliflozin | Randomized, Double Blind |
T2D With Cardiovascular Disease | 8,246 | 206 weeks | 19% Not statistically significant |
Safety and adverse effects of SGLT2 inhibitors
Adverse effects need to be taken in count when using SGLT2 inhibitors as a pharmacologic therapy for type 2 diabetes patients, as they impact quality of life [36]. Side effects of SGLT2 inhibitors include: Genital infections, orthostatic hypotension, polyuria, euglycemic diabetic ketoacidosis, electrolyte disturbance, bone fractures or amputations of the toes/feet (Table 2) [37].
| Table 2: Incidence of adverse effects of SGLT2 inhibitors in randomized clinical trials. Relative Risk (RR) estimated from published data. +Incidence Relative Risk (IRR) estimated from published data. aGlomerular filtration rate barrier to 60 mL/min/1.73 m². bThe incidence rate were only notified in the CANVAS trial (4330 participants). cOnly important hypoglycemic effects were reported. donly mycotic genital infection was reported. NA: None applied because the number of events was low [32-35]. | ||||
| Study | EMPA-REG OUTCOME | CANVAS | DECLARE-TIMI 58 | CREDENCE |
| SGLT-2 | Empagliflozin | Canagliflozin | Dapagliflozin | Canagliflozin |
| Control | Placebo | Placebo | Placebo | Placebo |
| Median follow-up (years) | 3.1 | 2.4 | 4.2 | 2.62 |
| Number of Patients (drug vs. placebo) | 4687 vs 2333 | 5749 vs 4347 | 8582 vs 8578 | 2202 vs 2199 |
| Hypoglicemia | No Difference | No Difference | Inferior (RR=0.7)*c | No Difference |
| Diabetic Ketoacidosis | NA | NA | Superior (RR=2.25)* | Superior (RR=10.99; IRR=11.00)*+ |
| Urinary Tract Infections | No Difference | No Difference | No Difference | No Difference |
| Urinary Tract Infections in Women | Inferior (RR=0.9)* | Non-reported | Non-reported | Non-reported |
| Genital Infections | Superior (RR=3.57)* | Non-reported | Superior (RR=1.33)* | Superior (RR=3.84)*+d |
| Urinary Tract Infections in Men | Superior (RR=3.34)* | Superior (IRR=3.23)+ | Non-reported | Superior (RR=9.51; IRR=9.33)*+d |
| Hypovolemia | No Difference | Superior (IRR=1.41)+b | No Difference | No Difference |
| Acute Kidney Disease | Inferior RR=0.61)* | No Difference | Inferior (RR=0.71)* | No Difference |
| Amputation | Non-reported | Superior (IRR=1.85)+ | No Difference | No Difference |
In general, the pharmacokinetics of these medications are altered with the presence of chronic kidney disease; however, in patients with mild CKD, the lowering glucose capacity and safety of SGLT2 inhibitors are similar that in patients with normal renal function [29]. Given this, it is necessary to evaluate renal function previous to the initiation of treatment, adjusting the necessities based on GFR. The threshold varies country to country; howeve,r as a general rule, SGLT2 inhibitors can be used safely in patients with an EGFR more than 30 mL/min/1.73 m² [3,29].
Several studies have demonstrated that glucosuria induced by SGLT2 inhibitors increases the risk of developing genital infections (balanitis and vulvovaginitis), with an increased risk in women more than men [38–40]. However, there has not been a dose-dependent correlation with these infections [41].
It is worse to mention the glucosuria induced by SGLT2 inhibitors this paradoxically associated with renal toxicity, given uric acid crystal deposition, leading to release of inflammatory mediators and reactive oxygen species, cytokine production, local tubular damage and electrolyte imbalances, involving mainly potassium and magnesium [42]. Similarly, it is worth to mention that the FDA warned about the risk of euglycemic ketoacidosis in patients treated with SGLT2 inhibitors [37]. This report came out based on the results of the EMPA-REG-OUTCOME and the CANVAS trials, which informed that SGLT2 inhibitors, particularly empagliflozin, increases the risk of ketoacidosis in patients with type 2 diabetes [36].
The CANVAS study informed that the patient’s treated with canagliflozin had a higher risk of mechanical bone fractures, given that SGLT2 inhibitors can exert adverse effects on bone physiology, by increasing the concentrations of PTH and the induction of fibroblast 23 growth factor (which seems to exist in these medications). It is necessary to use these with caution in people with high risk of fractures or high risk of falls (including those with hypotension) [37,41]. Additionally, different from the CREDENCE and the DECLARE-TIMI-58, this study demonstrated the canagliflozin and a dose of 100 or 200 mg increased the risk of amputation, mainly of the toes and metatarsals, with a predisposition on patients with previous amputations [24].
Equilibrating their adverse effects versus the benefits and security profile, it is important to evaluate each patient individually to have more integral control of the disease, decreasing the possible side effects produced by these medications.
Clinical use of SGLT2 inhibitors
FDA approved canagliflozin for the event of type 2 diabetes in 2013, SGLT2 inhibitors have gained approval worldwide [3,41]. Currently, the use of SGLT2 inhibitors in diabetic kidney disease has become evident, given that 30% to 35% of patients with type 2 diabetes suffer from this complication [9].
The pharmacologic properties of SGLT2 inhibitors have categorized them as an alternative use in favor of kidney health in medicated patients, demonstrating a reduction in the progression of diabetic kidney disease with nephro protective effects [9,29,42]. SGLT2 inhibitors have demonstrated these effects independently from cardiovascular morbidity including patients with chronic kidney disease with a GFR of less than 30 mL/min/1.73 m², reason why it is recommended to use empagliflozin and canagliflozin when the GFR is more than 30 mL/min/1.73 m²; dapagliflozin is recommended when the GFR is less than 60 mL/min/1.73 m² [37,42]. The intrarenal hemodynamic effects are the reason of the improved kidney function, product of the decrease in plasma volume and blood pressure, reduction of 30 to 40% in albuminuria and the modification of inflammatory processes [3,29,36,38]. The EMPA-REG OUTCOME and CANVAS studies demonstrate a decrease in the hyperfiltration and intraglomerular pressure in patients that participated in the studies, which may suggest a decrease in the rate of progression of diabetic kidney disease or even in nondiabetic patients with CKD [9,36,37]. Based on these reports, the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommend the use of pharmacologic therapy with SGLT2 inhibitors as the drug of choice in glucose lowering therapy in patients with cardiovascular disease or pre-existing chronic kidney disease [43].
SGLT2 inhibitors also been used in type 1 diabetes. Currently, Sotaglifozin is approved for use in patients with type 1 diabetes in Japan, given its benefit in reducing hemoglobin A1c levels, decreasing the doses of insulin, weight loss and a lower risk of symptomatic hypoglycemia [44]. We are waiting for the official publishing of the trials DAPA CKD and EMPA-KIDNEY, to confirm what the SGLT2 inhibitors have demonstrated so far, including protective cardiovascular and renal properties additional to blood glucose control.
SGLT2 inhibitors are of particular interest for the nephrology community. Diabetic kidney disease is the main cause of renal replacement therapy initiation worldwide, the nephro protection observed in patients with type 2 diabetes with these medications is of critical importance. The knowledge of the implied mechanisms and the nephro protective effects of these medications have increased progressively, with solid existing evidence backed up by the aforementioned clinical trials. It is expected that the renal effects of these medications encourage clinicians to utilize them more frequently in the care of type 2 diabetes. Of course, it is also worth considering the adverse effects, the costs, alternative agents and the individual characteristics of each patient. The findings of the current and future clinical trials will continue to help clarify the role in the mechanism of SGLT2 inhibitors in the long-term protection of kidney function in patients with type 2 diabetes.
- De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, et al. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: Targeting the renal tubule in diabetes. Am J Kidney Dis. 2014; 64: 16–24. PubMed: https://pubmed.ncbi.nlm.nih.gov/24673844
- Kattyuska V, Daniela M, Manuel TR, Gema R, Rafel C, et al. Complicaciones microvasculares de la diabetes. Rev Venez Endocrinol Metab. 2012; 10(Suppl 1): 111-137. Disponible en: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1690-31102012000400014&lng=es.
- Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium–glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019; 68: 248–257. PubMed: https://pubmed.ncbi.nlm.nih.gov/30665953/
- Rosas Guzmán J, García Rubí E, Gómez Pérez F, Calles J. Prevención, diagnóstico y tratamiento temprano de la Nefropatía Diabética. Revista ALAD (Revista On-line) 2009. 2016; 26. Consesos ALAD. 2011; 16: 106–114.
- Barutta F, Bernardi S, Gargiulo G, Gruden G. SGLT2 inhibition to address the unmet needs in diabetic nephropathy. Diabetes Metab Res Rev. 2019; 35: e3171. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6849789/
- Torres A, Zacarías R. Nefropatía diabética. Rev Hosp Gral Dr. M Gea González. 2002; 5: 24-32.
- Espa G. Tema monográfico Fisiopatología de la nefropatía diabética. 2008. https://www.revistanefrologia.com/es-fisiopatologia-nefropatia-diabetica-articulo-X1888970008000118
- Kawanami D, Matoba K, Takeda Y, Nagai Y, Akamine T, et al. SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int J Mol Sci. 2017; 18: 1083. PubMed: https://pubmed.ncbi.nlm.nih.gov/28524098
- Maltese G, Abou-saleh A, Gnudi L, Karalliedde J. Preventing diabetic renal disease: the potential reno-protective effects of SGLT2 inhibitors. Br J Diabetes Vasc Dis. 2015; 15: 114-118.
- Gonzalez DE, Foresto RD, Ribeiro AB. SGLT-2 inhibitors in diabetes : a focus on renoprotection. Rev Assoc Med Bras. 2020; 66(Suppl 1): 17–24. PubMed: https://pubmed.ncbi.nlm.nih.gov/31939531/
- Washburn W. Case History: ForxigaTM (Dapagliflozin), a Potent Selective SGLT2 Inhibitor for Treatment of Diabetes. Annual Reports in Medicinal Chemistry. 2014; 49.
- Meng W, Ellsworth B, Nirschl A, McCann P, Patel M, et al. Discovery of Dapagliflozin: A Potent, Selective Renal Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes. J Med Chem. 2008; 51:1145–1149. PubMed: https://pubmed.ncbi.nlm.nih.gov/18260618/
- Choi C. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors from Natural Products: Discovery of Next-Generation Antihyperglycemic Agents. Molecules. 2016; 21: 1136. PubMed: https://pubmed.ncbi.nlm.nih.gov/27618891/
- Cai W, Jiang L, Xie Y, Liu Y, Liu W, et al. Design of SGLT2 Inhibitors for the Treatment of Type 2 Diabetes: A History Driven by Biology to Chemistry. Medicinal Chemistry. 2015; 11: 317-328. PubMed: https://pubmed.ncbi.nlm.nih.gov/25557661/
- White J. Apple Trees to Sodium Glucose Co-Transporter Inhibitors: A Review of SGLT2 Inhibition. Clinical Diabetes. 2010; 28.
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, et al. Discovery of Canagliflozin, a Novel C-Glucoside with Thiophene Ring, as Sodium-Dependent Glucose Cotransporter 2 Inhibitor for the Treatment of Type 2 Diabetes Mellitus1. J. Med. Chem. 2010; 53: 6355–6360. PubMed: https://pubmed.ncbi.nlm.nih.gov/20690635/
- Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018; 61: 2079–2086. PubMed: https://pubmed.ncbi.nlm.nih.gov/30132033/
- Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, et al. Discovery of Canagliflozin, a Novel C-Glucoside with Thiophene Ring, as Sodium-Dependent Glucose Cotransporter 2 Inhibitor for the Treatment of Type 2 Diabetes Mellitus1. J. Med. Chem. 2010; 53: 6355–6360. PubMed: https://pubmed.ncbi.nlm.nih.gov/20690635/
- Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes, Obesity and Metabolism. 2012; 14: 83–90. PubMed: https://pubmed.ncbi.nlm.nih.gov/21985634
- Rossato M, Busetto L. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016; 39(Suppl 2): S165-S171. PubMed: https://pubmed.ncbi.nlm.nih.gov/27440829/
- Dekkers CCJ. Sodium-glucose cotransporter 2 inhibitors: extending the indication to non-diabetic kidney disease? Nephrol Dial Transplant. 2020; 35: i33–i42. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6993196/
- Rola N, Elias K, Farid N, Inbal D, Farber E, et al. Sodium-Glucose Transporter Inhibitors and ŝĂbĞtic Nephropathy in Humans and Animal Model. J Clin Exp Nephrol. 2018; 3: 10.
- Cowie MR. Fisher M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020. PubMed: https://pubmed.ncbi.nlm.nih.gov/32665641/
- Górriz JL, Navarro-González JF, Ortiz A, Vergara A, Nuñez J, et al. Sodium-glucose cotransporter 2 inhibition: towards an indication to treat diabetic kidney disease, Nephrology Dialysis Transplantation. 2020. 35 (Suppl 1): i13–i23. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6993197/
- Yaribeygi H, Simental-mendía LE, Banach M, Bo S, Sahebkar A. Biomedicine & Pharmacotherapy The major molecular mechanisms mediating the renoprotective e ff ects of SGLT2 inhibitors : An update. 2019; 120: 109526.
- Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, et al. Articles SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. LANCET Diabetes Endocrinol. 2019; 7: 845-854. PubMed: https://pubmed.ncbi.nlm.nih.gov/31495651/
- Mikhail N. SGLT2 Inhibitors for Treatment of Diabetic Nephropathy. Curre Res Diabetes Obes J. 2019; 12: 555839.
- Gilbert RE. Sodium – glucose linked transporter-2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int. 2013; 86: 693–700. PubMed: https://pubmed.ncbi.nlm.nih.gov/24257692/
- Davidson JA. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med. 2019. 131: 251-260. PubMed: https://pubmed.ncbi.nlm.nih.gov/30929540/
- Eren Z, Gunal MY, Ari E, Coban J, Cakalagaoglu F, et al. Pleiotropic and renoprotective effects of erythropoietin β on experimental diabetic nephropathy model. Nephron. 2016; 132: 292–300. PubMed: https://pubmed.ncbi.nlm.nih.gov/26938976/
- Tsuruya K, Yoshida H, Suehiro T, Fujisaki K, Masutani K, et al. Erythropoiesis-stimulating agent slows the progression of chronic kidney disease: A possibility of a direct action of erythropoietin. Ren Fail. 2016; 38: 390–396. PubMed: https://pubmed.ncbi.nlm.nih.gov/26822074/
- Zinman B, Wanner C, Lachin J, Fitchett D, Bluhmki E, Hantel S et al. Empaglifozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015; 373: 2117-2128. PubMed: https://pubmed.ncbi.nlm.nih.gov/26378978/
- Neal B, Perkovic V, Mahaffey K, Zeeuw D, Fulcher G, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377: 644–657. PubMed: https://pubmed.ncbi.nlm.nih.gov/28605608/
- Perkovic V, Jardine M, Neal B, Bompoint S, Heerspink H, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380: 2295–2306. PubMed: https://pubmed.ncbi.nlm.nih.gov/30990260/
- Wiviott S, Raz I, Bonaca M, Mosenzon O, Kato E, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380: 347–357. PubMed: https://pubmed.ncbi.nlm.nih.gov/30415602/
- Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015; 100: 2849-2852. PubMed: https://pubmed.ncbi.nlm.nih.gov/26086329/
- Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017; 136: 1643-1658. PubMed: https://pubmed.ncbi.nlm.nih.gov/29061576/
- Tsimihodimos V, Filippatos TD, Elisaf MS. SGLT2 INHIBITORS AND THE KIDNEY: EFFECTS AND MECHANISMS, Diabetes and Metabolic Syndrome: Clin Res Rev. 2018.
- Verma S. McMurray J. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018; 61: 2108-2117. PubMed: https://pubmed.ncbi.nlm.nih.gov/30132036/
- Shiba K, Tsuchiya K, Komiya C, et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018; 8: 2362. PubMed: https://pubmed.ncbi.nlm.nih.gov/29402900/
- Scheen AJ. An update on the safety of SGLT2 inhibitors, Expert Opin Drug Saf. 2019; 18: 295-311. PubMed: https://pubmed.ncbi.nlm.nih.gov/30933547/
- Hecking M, Jenssen T. Considerations for SGLT2 inhibitor use in post-transplantation diabetes. Nat Rev Nephrol. 2019; 15: 525-526. PubMed: https://pubmed.ncbi.nlm.nih.gov/31235880/
- Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41: 2669–2701. PubMed: https://pubmed.ncbi.nlm.nih.gov/30291106/
- Rico J, Enfermedad Renal Diabética, Capitulo 15. Nefrología Básica. Asociación Colombiana de Nefrología e Hipertensión arterial. http://asocolnef.com/formacion-2/formacion/actualizacion-libro-nefrologia-basica-2/
- Lopera Vargas JM, Rico Fontalvo JE, Melgarejo E, Castillo Barrios GA, Ramírez Rincó A, et al. Efecto de terapias farmacológicas para el control glicémico en pacientes con diabetes mellitus tipo 2 en los desenlaces vasculares. Rev Colomb Nefrol. 2020; 7: 44-59.
- Nuffer W, Williams B, Trujillo J. A review of sotagliflozin for use in type 1 diabetes. Ther Adv Endocrinol Metab. 2019; 10: 2042018819890527. PubMed: https://pubmed.ncbi.nlm.nih.gov/31807264/
- Burns, K, Cherney, D. Renal Angiotensinogen and Sodium-Glucose Cotransporter-2 Inhibition: Insights from Experimental Diabetic Kidney Disease. Am J Nephrol. 2019; 49: 328–330. PubMed: https://www.karger.com/Article/Abstract/501081
- Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Scientific Reports. 2019; 9: 14754.
- Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019; 4: e123130.